The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation
- PMID: 23623682
- PMCID: PMC3654024
- DOI: 10.1016/j.molcel.2013.03.021
The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation
Abstract
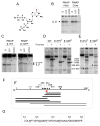
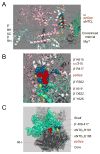
The global regulatory nucleotide ppGpp ("magic spot") regulates transcription from a large subset of Escherichia coli promoters, illustrating how small molecules can control gene expression promoter-specifically by interacting with RNA polymerase (RNAP) without binding to DNA. However, ppGpp's target site on RNAP, and therefore its mechanism of action, has remained unclear. We report here a binding site for ppGpp on E. coli RNAP, identified by crosslinking, protease mapping, and analysis of mutant RNAPs that fail to respond to ppGpp. A strain with a mutant ppGpp binding site displays properties characteristic of cells defective for ppGpp synthesis. The binding site is at an interface of two RNAP subunits, ω and β', and its position suggests an allosteric mechanism of action involving restriction of motion between two mobile RNAP modules. Identification of the binding site allows prediction of bacterial species in which ppGpp exerts its effects by targeting RNAP.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Bacterial transcription: Finding the magic spot on RNAP.Nat Rev Microbiol. 2013 Jul;11(7):429. doi: 10.1038/nrmicro3053. Epub 2013 Jun 10. Nat Rev Microbiol. 2013. PMID: 23748340 No abstract available.
References
-
- Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG. Structural basis for transcription regulation by alarmone ppGpp. Cell. 2004;117:299–310. - PubMed
-
- Artsimovitch I, Svetlov V, Murakami KS, Landick R. Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. J Biol Chem. 2003;278:12344–12355. - PubMed
-
- Barker MM, Gaal T, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. II Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol. 2001a;305:689–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

