The mechanism of E. coli RNA polymerase regulation by ppGpp is suggested by the structure of their complex
- PMID: 23623685
- PMCID: PMC3677725
- DOI: 10.1016/j.molcel.2013.03.020
The mechanism of E. coli RNA polymerase regulation by ppGpp is suggested by the structure of their complex
Abstract
Guanosine tetraphosphate (ppGpp) is an alarmone that enables bacteria to adapt to their environment. It has been known for years that ppGpp acts directly on RNA polymerase (RNAP) to alter the rate of transcription, but its exact target site is still under debate. Here we report a crystal structure of Escherichia coli RNAP holoenzyme in complex with ppGpp at 4.5 Å resolution. The structure reveals that ppGpp binds at an interface between the shelf and core modules on the outer surface of RNAP, away from the catalytic center and the nucleic acid binding path. Bound ppGpp connects these two pivotal modules that may restrain the opening of the RNAP cleft. A detailed mechanism of action of ppGpp is proposed in which ppGpp prevents the closure of the active center that is induced by the binding of NTP, which could slow down nucleotide addition cycles and destabilize the initial transcription complexes.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
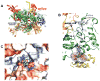


Comment in
-
Bacterial transcription: Finding the magic spot on RNAP.Nat Rev Microbiol. 2013 Jul;11(7):429. doi: 10.1038/nrmicro3053. Epub 2013 Jun 10. Nat Rev Microbiol. 2013. PMID: 23748340 No abstract available.
References
-
- Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG. Structural basis for transcription regulation by alarmone ppGpp. Cell. 2004;117:299–310. - PubMed
-
- Barker MM, Gaal T, Josaitis CA, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. I Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol. 2001a;305:673–688. - PubMed
-
- Barker MM, Gaal T, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. II Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol. 2001b;305:689–702. - PubMed
-
- Buglino J, Shen V, Hakimian P, Lima CD. Structural and biochemical analysis of the Obg GTP binding protein. Structure. 2002;10:1581–1592. - PubMed
-
- Cashel M, Gentry DR, Hernandez VH, Vinella D. In: The stringent response Escherichia coli and Salmonella. Neidhardt FC, editor. ASM Press; Washington, DC: 1996. pp. 1458–1496.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

