GABAA receptor modulation by piperine and a non-TRPV1 activating derivative
- PMID: 23623790
- PMCID: PMC3776227
- DOI: 10.1016/j.bcp.2013.04.017
GABAA receptor modulation by piperine and a non-TRPV1 activating derivative
Abstract
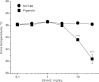
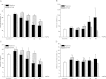
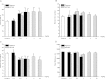
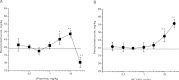
The action of piperine (the pungent component of pepper) and its derivative SCT-66 ((2E,4E)-5-(1,3-benzodioxol-5-yl))-N,N-diisobutyl-2,4-pentadienamide) on different gamma-aminobutyric acid (GABA) type A (GABA(A)) receptors, transient-receptor-potential-vanilloid-1 (TRPV1) receptors and behavioural effects were investigated. GABA(A) receptor subtypes and TRPV1 receptors were expressed in Xenopus laevis oocytes. Modulation of GABA-induced chloride currents (I(GABA)) by piperine and SCT-66 and activation of TRPV1 was studied using the two-microelectrode-voltage-clamp technique and fast perfusion. Their effects on explorative behaviour, thermoregulation and seizure threshold were analysed in mice. Piperine acted with similar potency on all GABA(A) receptor subtypes (EC₅₀ range: 42.8±7.6 μM (α₂β₂)-59.6±12.3 μM (α₃β₂). I(GABA) modulation by piperine did not require the presence of a γ(2S)-subunit, suggesting a binding site involving only α and β subunits. I(GABA) activation was slightly more efficacious on receptors formed from β(2/3) subunits (maximal I(GABA) stimulation through α₁β₃ receptors: 332±64% and α₁β₂: 271±36% vs. α₁β₁: 171±22%, p<0.05) and α₃-subunits (α₃β₂: 375±51% vs. α₅β₂:136±22%, p<0.05). Replacing the piperidine ring by a N,N-diisobutyl residue (SCT-66) prevents interactions with TRPV1 and simultaneously increases the potency and efficiency of GABA(A) receptor modulation. SCT-66 displayed greater efficacy on GABA(A) receptors than piperine, with different subunit-dependence. Both compounds induced anxiolytic, anticonvulsant effects and reduced locomotor activity; however, SCT-66 induced stronger anxiolysis without decreasing body temperature and without the proconvulsive effects of TRPV1 activation and thus may serve as a scaffold for the development of novel GABA(A) receptor modulators.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Efficient modulation of γ-aminobutyric acid type A receptors by piperine derivatives.J Med Chem. 2014 Jul 10;57(13):5602-19. doi: 10.1021/jm5002277. Epub 2014 Jun 27. J Med Chem. 2014. PMID: 24905252 Free PMC article.
-
HPLC-based activity profiling: discovery of piperine as a positive GABA(A) receptor modulator targeting a benzodiazepine-independent binding site.J Nat Prod. 2010 Feb 26;73(2):185-91. doi: 10.1021/np900656g. J Nat Prod. 2010. PMID: 20085307 Free PMC article.
-
Developing piperine towards TRPV1 and GABAA receptor ligands--synthesis of piperine analogs via Heck-coupling of conjugated dienes.Org Biomol Chem. 2015 Jan 28;13(4):990-4. doi: 10.1039/c4ob02242d. Epub 2014 Dec 1. Org Biomol Chem. 2015. PMID: 25438036
-
Preparation, pungency and bioactivity transduction of piperine from black pepper (Piper nigrum L.): A comprehensive review.Food Chem. 2024 Oct 30;456:139980. doi: 10.1016/j.foodchem.2024.139980. Epub 2024 Jun 4. Food Chem. 2024. PMID: 38850607 Review.
-
Piper nigrum and piperine: an update.Phytother Res. 2013 Aug;27(8):1121-30. doi: 10.1002/ptr.4972. Epub 2013 Apr 29. Phytother Res. 2013. PMID: 23625885 Review.
Cited by
-
A distinct structural mechanism underlies TRPV1 activation by piperine.Biochem Biophys Res Commun. 2019 Aug 20;516(2):365-372. doi: 10.1016/j.bbrc.2019.06.039. Epub 2019 Jun 15. Biochem Biophys Res Commun. 2019. PMID: 31213294 Free PMC article.
-
Efficient modulation of γ-aminobutyric acid type A receptors by piperine derivatives.J Med Chem. 2014 Jul 10;57(13):5602-19. doi: 10.1021/jm5002277. Epub 2014 Jun 27. J Med Chem. 2014. PMID: 24905252 Free PMC article.
-
S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): A Novel Cinnamamide Derivative with Anticonvulsant Activity in Animal Models of Seizures and Epilepsy.Int J Mol Sci. 2020 Jun 19;21(12):4372. doi: 10.3390/ijms21124372. Int J Mol Sci. 2020. PMID: 32575479 Free PMC article.
-
Interference of TRPV1 function altered the susceptibility of PTZ-induced seizures.Front Cell Neurosci. 2015 Feb 10;9:20. doi: 10.3389/fncel.2015.00020. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25713512 Free PMC article.
-
A Systematic Review of the Anti-seizure and Antiepileptic Effects and Mechanisms of Piperine.Cent Nerv Syst Agents Med Chem. 2025;25(2):143-156. doi: 10.2174/0118715249297934240630111059. Cent Nerv Syst Agents Med Chem. 2025. PMID: 39082167
References
-
- Szallasi A. Piperine: researchers discover new flavor in an ancient spice. Trends Pharmacol Sci. 2005;26:437–439. - PubMed
-
- Di Marzo V., Gobbi G., Brain Szallasi A. TRPV1: a depressing TR(i)P down memory lane. Trends Pharmacol Sci. 2008;29:594–600. - PubMed
-
- Gunthorpe M.J., Chizh B.A. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14:56–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

