Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model
- PMID: 23624001
- PMCID: PMC3710969
- DOI: 10.1016/j.jhep.2013.04.014
Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model
Abstract
Background & aims: Sinusoidal obstruction syndrome (SOS) following oxaliplatin based chemotherapy can have a significant impact on post-operative outcome following resection of colorectal liver metastases. To date no relevant experimental models of oxaliplatin induced SOS have been described. The aim of this project was to establish a rodent model which could be utilised to investigate mechanisms underlying SOS to aid the development of therapeutic strategies.
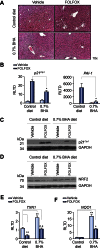
Methods: C57Bl/6 mice, maintained on a purified diet, were treated with intra-peritoneal FOLFOX (n=10), or vehicle (n=10), weekly for five weeks and culled one week following final treatment. Sections of the liver and spleen were fixed in formalin and paraffin embedded for histological analysis. The role of oxidative stress on experimental-induced SOS was determined by dietary supplementation with butylated hydroxyanisole and N-acetylcysteine.
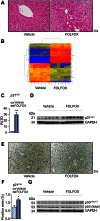
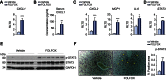
Results: FOLFOX treatment was associated with the development of sinusoidal dilatation and hepatocyte atrophy on H&E stained sections of the liver in keeping with SOS. Immunohistochemistry for p21 demonstrated the presence of replicative senescence within the sinusoidal endothelium. FOLFOX induced endothelial damage leads to a pro-thrombotic state within the liver associated with upregulation of PAI-1 (p<0.001), vWF (p<0.01) and Factor X (p<0.001), which may contribute to the propagation of liver injury. Dietary supplementation with the antioxidant BHA prevented the development of significant SOS.
Conclusions: We have developed the first reproducible model of chemotherapy induced SOS that reflects the pathogenesis of this disease in patients. It appears that the use of antioxidants alongside oxaliplatin based chemotherapy may be of value in preventing the development of SOS in patients with colorectal liver metastases.
Keywords: ALP; ALT; AST; BHA; CRLM; CXCL1/2; Chemotherapy induced liver injury; Colorectal liver metastases; GAPDH; H&E; HPF; IL-6; MCP1; N-acetylcysteine; NAC; NAD(P)H dehydrogenase 1; NQO1; NRF2; Oxaliplatin; PAI-1; PAR 1/2; PCNA; SOS; STAT3; Sinusoidal obstruction syndrome; TXN1; VEGF-A/B/C; VEGFR-1/2; alanine aminotransferase; alkaline phosphatase; aspartate aminotransferase; butylated hydroxyanisole; chemokine (C-X-C motif) ligand 1/2; colorectal liver metastases; gyceraldehyde 3-phosphate dehydrogenase; haematoxylin and eosin; high powered field; i.p.; interleukin 6; intraperitoneal; monocyte chemotactic protein-1; nuclear factor (erythroid-derived 2)-like 2; phosphorylated form of the H2A histone family, member X; plasminogen activatior inhibitor 1; proliferating cell nuclear antigen; protease activated receptor 1/2; signal transducer and activator of transcription 3; sinusoidal obstruction syndrome; thioredoxin 1; vWF; vascular endothelial growth factor A/B/C; vascular endothelial growth factor receptor ½; von Willebrand factor; γH2AX.
Copyright © 2013 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. [see comment] - PubMed
-
- Rubbia-Brandt L., Audard V., Sartoretti P., Roth A.D., Brezault C., Le Charpentier M. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. - PubMed
-
- Hubert C., Fervaille C., Sempoux C., Horsmans Y., Humblet Y., Machiels J.P. Prevalence and clinical relevance of pathological hepatic changes occurring after neoadjuvant chemotherapy for colorectal liver metastases. Surgery. 2010;147:185–194. - PubMed
-
- Vauthey J.N., Pawlik T.M., Ribero D., Wu T.T., Zorzi D., Hoff P.M. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- NC/K000748/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- WT084961MA/WT_/Wellcome Trust/United Kingdom
- G0900535/MRC_/Medical Research Council/United Kingdom
- WT087961MA/WT_/Wellcome Trust/United Kingdom
- WT090974MA/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

