Defense genes missing from the flight division
- PMID: 23624185
- PMCID: PMC7172724
- DOI: 10.1016/j.dci.2013.04.010
Defense genes missing from the flight division
Abstract
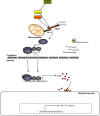
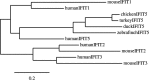
Birds have a smaller repertoire of immune genes than mammals. In our efforts to study antiviral responses to influenza in avian hosts, we have noted key genes that appear to be missing. As a result, we speculate that birds have impaired detection of viruses and intracellular pathogens. Birds are missing TLR8, a detector for single-stranded RNA. Chickens also lack RIG-I, the intracellular detector for single-stranded viral RNA. Riplet, an activator for RIG-I, is also missing in chickens. IRF3, the nuclear activator of interferon-beta in the RIG-I pathway is missing in birds. Downstream of interferon (IFN) signaling, some of the antiviral effectors are missing, including ISG15, and ISG54 and ISG56 (IFITs). Birds have only three antibody isotypes and IgD is missing. Ducks, but not chickens, make an unusual truncated IgY antibody that is missing the Fc fragment. Chickens have an expanded family of LILR leukocyte receptor genes, called CHIR genes, with hundreds of members, including several that encode IgY Fc receptors. Intriguingly, LILR homologues appear to be missing in ducks, including these IgY Fc receptors. The truncated IgY in ducks, and the duplicated IgY receptor genes in chickens may both have resulted from selective pressure by a pathogen on IgY FcR interactions. Birds have a minimal MHC, and the TAP transport and presentation of peptides on MHC class I is constrained, limiting function. Perhaps removing some constraint, ducks appear to lack tapasin, a chaperone involved in loading peptides on MHC class I. Finally, the absence of lymphotoxin-alpha and beta may account for the observed lack of lymph nodes in birds. As illustrated by these examples, the picture that emerges is some impairment of immune response to viruses in birds, either a cause or consequence of the host-pathogen arms race and long evolutionary relationship of birds and RNA viruses.
Keywords: Chicken; Duck; Lymph node; Major Histocompatibility Complex; RIG-I; TLR8; pathway.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Gene expression profile after activation of RIG-I in 5'ppp-dsRNA challenged DF1.Dev Comp Immunol. 2016 Dec;65:191-200. doi: 10.1016/j.dci.2016.07.009. Epub 2016 Jul 20. Dev Comp Immunol. 2016. PMID: 27450445
-
Association of RIG-I with innate immunity of ducks to influenza.Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5913-8. doi: 10.1073/pnas.1001755107. Epub 2010 Mar 22. Proc Natl Acad Sci U S A. 2010. PMID: 20308570 Free PMC article.
-
Chicken interferons, their receptors and interferon-stimulated genes.Dev Comp Immunol. 2013 Nov;41(3):370-6. doi: 10.1016/j.dci.2013.05.020. Epub 2013 Jun 7. Dev Comp Immunol. 2013. PMID: 23751330 Review.
-
IRF7 Is Involved in Both STING and MAVS Mediating IFN-β Signaling in IRF3-Lacking Chickens.J Immunol. 2019 Oct 1;203(7):1930-1942. doi: 10.4049/jimmunol.1900293. Epub 2019 Jul 31. J Immunol. 2019. PMID: 31366714
-
Pattern Recognition Receptor Signaling and Innate Responses to Influenza A Viruses in the Mallard Duck, Compared to Humans and Chickens.Front Cell Infect Microbiol. 2020 May 12;10:209. doi: 10.3389/fcimb.2020.00209. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32477965 Free PMC article. Review.
Cited by
-
A new chromosome-scale duck genome shows a major histocompatibility complex with several expanded multigene families.BMC Biol. 2024 Feb 5;22(1):31. doi: 10.1186/s12915-024-01817-0. BMC Biol. 2024. PMID: 38317190 Free PMC article.
-
Avian Influenza NS1 Proteins Inhibit Human, but Not Duck, RIG-I Ubiquitination and Interferon Signaling.J Virol. 2022 Sep 28;96(18):e0077622. doi: 10.1128/jvi.00776-22. Epub 2022 Sep 7. J Virol. 2022. PMID: 36069546 Free PMC article.
-
Duck karyopherin α4 (duKPNA4) is involved in type I interferon expression and the antiviral response against Japanese encephalitis virus.Dev Comp Immunol. 2020 Mar;104:103535. doi: 10.1016/j.dci.2019.103535. Epub 2019 Nov 5. Dev Comp Immunol. 2020. PMID: 31697956 Free PMC article.
-
Innate Immune Responses to Avian Influenza Viruses in Ducks and Chickens.Vet Sci. 2019 Jan 10;6(1):5. doi: 10.3390/vetsci6010005. Vet Sci. 2019. PMID: 30634569 Free PMC article. Review.
-
A chromosome-level genome assembly for the Silkie chicken resolves complete sequences for key chicken metabolic, reproductive, and immunity genes.Commun Biol. 2023 Dec 6;6(1):1233. doi: 10.1038/s42003-023-05619-y. Commun Biol. 2023. PMID: 38057566 Free PMC article.
References
-
- Au W.C., Moore P.A., Lowther W., Juang Y.T., Pitha P.M. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA. 1995;92:11657–11661. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

