Foxg1 is required to limit the formation of ciliary margin tissue and Wnt/β-catenin signalling in the developing nasal retina of the mouse
- PMID: 23624311
- PMCID: PMC3722486
- DOI: 10.1016/j.ydbio.2013.04.017
Foxg1 is required to limit the formation of ciliary margin tissue and Wnt/β-catenin signalling in the developing nasal retina of the mouse
Abstract
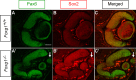
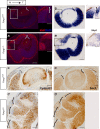
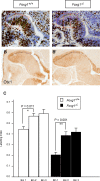
The ciliary margin (CM) develops in the peripheral retina and gives rise to the iris and the ciliary body. The Wnt/β-catenin signalling pathway has been implicated in ciliary margin development. Here, we tested the hypothesis that in the developing mouse retina Foxg1 is responsible for suppressing the Wnt/β-catenin pathway and restricting CM development. We showed that there is excess CM tissue in Foxg1(-/-) null embryos and this expansion is more pronounced in the nasal retina where Foxg1 normally shows its highest expression levels. Results on expression of a reporter allele for Wnt/β-catenin signalling and of Lef1, a target of Wnt/β-catenin signalling, displayed significant upregulation of this pathway in Foxg1(-/-) nulls at embryonic days 12.5 and 14.5. Interestingly, this upregulation was observed specifically in the nasal retina, where normally very few Wnt-responsive cells are observed. These results indicate a suppressive role of Foxg1 on this signalling pathway. Our results reveal a new role of Foxg1 in limiting CM development in the nasal peripheral retina and add a new molecular player in the developmental network involved in CM specification.
Keywords: Ciliary margin; Eye development; Foxg1; Mouse; Wnt/β-catenin signalling.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures








References
-
- Amae S., Fuse N., Yasumoto K., Sato S., Yajima I., Yamamoto H., Udono T., Durlu Y.K., Tamai M., Takahashi K., Shibahara S. Identification of a novel isoform of microphthalmia-associated transcription factor that is enriched in retinal pigment epithelium. Biochem. Biophys. Res. Commun. 1998;247:710–715. - PubMed
-
- Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505–7511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

