Hierarchy of orofacial rhythms revealed through whisking and breathing
- PMID: 23624373
- PMCID: PMC4159559
- DOI: 10.1038/nature12076
Hierarchy of orofacial rhythms revealed through whisking and breathing
Abstract
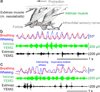
Whisking and sniffing are predominant aspects of exploratory behaviour in rodents. Yet the neural mechanisms that generate and coordinate these and other orofacial motor patterns remain largely uncharacterized. Here we use anatomical, behavioural, electrophysiological and pharmacological tools to show that whisking and sniffing are coordinated by respiratory centres in the ventral medulla. We delineate a distinct region in the ventral medulla that provides rhythmic input to the facial motor neurons that drive protraction of the vibrissae. Neuronal output from this region is reset at each inspiration by direct input from the pre-Bötzinger complex, such that high-frequency sniffing has a one-to-one relationship with whisking, whereas basal respiration is accompanied by intervening whisks that occur between breaths. We conjecture that the respiratory nuclei, which project to other premotor regions for oral and facial control, function as a master clock for behaviours that coordinate with breathing.
Figures