Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance
- PMID: 23624512
- PMCID: PMC3896394
- DOI: 10.1038/nn.3387
Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance
Abstract
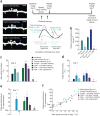
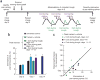
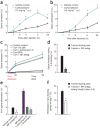
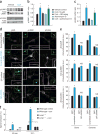
Excessive glucocorticoid exposure during chronic stress causes synapse loss and learning impairment. Under normal physiological conditions, glucocorticoid activity oscillates in synchrony with the circadian rhythm. Whether and how endogenous glucocorticoid oscillations modulate synaptic plasticity and learning is unknown. Here we show that circadian glucocorticoid peaks promote postsynaptic dendritic spine formation in the mouse cortex after motor skill learning, whereas troughs are required for stabilizing newly formed spines that are important for long-term memory retention. Conversely, chronic and excessive exposure to glucocorticoids eliminates learning-associated new spines and disrupts previously acquired memories. Furthermore, we show that glucocorticoids promote rapid spine formation through a non-transcriptional mechanism by means of the LIM kinase-cofilin pathway and increase spine elimination through transcriptional mechanisms involving mineralocorticoid receptor activation. Together, these findings indicate that tightly regulated circadian glucocorticoid oscillations are important for learning-dependent synaptic formation and maintenance. They also delineate a new signaling mechanism underlying these effects.
Figures


Comment in
-
Learning and memory: Learning with peaks and troughs.Nat Rev Neurosci. 2013 Jun;14(6):380. doi: 10.1038/nrn3515. Epub 2013 May 9. Nat Rev Neurosci. 2013. PMID: 23657555 No abstract available.
-
Learning to deal with life's ups and downs.Nat Neurosci. 2013 Jun;16(6):658-9. doi: 10.1038/nn.3400. Nat Neurosci. 2013. PMID: 23712065 Free PMC article.
References
-
- de Kloet ER, Vreugdenhil E, Oitzl MS, Jöels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. - PubMed
-
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. - PubMed
-
- Lupien SJ, et al. Cortisol levels during human aging predict hippocampal atrophy and memory defcits. Nat Neurosci. 1998;1:69–73. - PubMed
-
- de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

