Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core
- PMID: 23624852
- PMCID: PMC3742574
- DOI: 10.1007/s00213-013-3082-0
Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core
Abstract
Rationale: Phasic dopamine (DA) signaling underlies reward learning. Cholinergic and glutamatergic inputs into the ventral tegmental area (VTA) are crucial for modulating burst firing activity and subsequent phasic DA release in the nucleus accumbens (NAc), but the specific VTA nicotinic receptor subtypes that regulate phasic DA release have not been identified.
Objective: The goal was to determine the role of VTA N-methyl-D-aspartate receptors (NMDARs) and specific subtypes of nicotinic acetylcholine receptors (nAChRs) in regulating phasic DA release in the NAc core.
Methods: Fast-scan cyclic voltammetry in anesthetized rats was combined with intra-VTA micro-infusion to evaluate the ability of glutamatergic and cholinergic drugs to modulate stimulated phasic DA release in the NAc core.
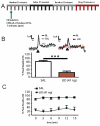
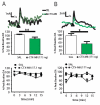
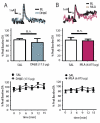
Results: VTA NMDAR blockade with AP-5 decreased, while VTA NMDAR activation with NMDA increased NAc peak phasic DA release. Intra-VTA administration of the nonspecific nAChR antagonist mecamylamine produced a persistent decrease in phasic DA release. Infusion of the α6-selective antagonist α-conotoxin MII (α-ctx MII) produced a robust, but transient decrease in phasic DA, whereas infusion of selective doses of either the α4β2-selective antagonist, dihydro-beta-erythroidine, or the α7 antagonist, methyllycaconitine, had no effect. Co-infusion of AP-5 and α-ctx MII produced a similar phasic DA decrease as either drug alone, with no additive effect.
Conclusions: The results suggest that VTA α6β2 nAChRs, but not α4β2 or α7 nAChRs, regulate phasic DA release in the NAc core and that VTA α6β2 nAChRs and NMDA receptors act at a common site or target to regulate NAc phasic DA signaling.
Figures


Similar articles
-
Differential role of ventral tegmental area acetylcholine and N-methyl-D-aspartate receptors in cocaine-seeking.Neuropharmacology. 2013 Dec;75:9-18. doi: 10.1016/j.neuropharm.2013.07.001. Epub 2013 Jul 11. Neuropharmacology. 2013. PMID: 23850572 Free PMC article.
-
Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice.Psychopharmacology (Berl). 2007 Feb;190(2):189-99. doi: 10.1007/s00213-006-0598-6. Epub 2006 Oct 24. Psychopharmacology (Berl). 2007. PMID: 17061109
-
Distinct effects of ventral tegmental area NMDA and acetylcholine receptor blockade on conditioned reinforcement produced by food-associated cues.Neuroscience. 2015 Aug 20;301:384-94. doi: 10.1016/j.neuroscience.2015.06.021. Epub 2015 Jun 17. Neuroscience. 2015. PMID: 26093048 Free PMC article.
-
Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology.Acta Pharmacol Sin. 2009 Jun;30(6):740-51. doi: 10.1038/aps.2009.63. Acta Pharmacol Sin. 2009. PMID: 19498417 Free PMC article. Review.
-
Tribute to: Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area [William Corrigall, Kathleen Coen and Laurel Adamson, Brain Res. 653 (1994) 278-284].Brain Res. 2016 Aug 15;1645:61-4. doi: 10.1016/j.brainres.2015.12.064. Epub 2016 Feb 8. Brain Res. 2016. PMID: 26867702 Review.
Cited by
-
Inversed Effects of Nav1.2 Deficiency at Medial Prefrontal Cortex and Ventral Tegmental Area for Prepulse Inhibition in Acoustic Startle Response.Mol Neurobiol. 2024 Feb;61(2):622-634. doi: 10.1007/s12035-023-03610-6. Epub 2023 Aug 31. Mol Neurobiol. 2024. PMID: 37650965
-
Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test.Behav Brain Res. 2015 Jul 15;288:54-62. doi: 10.1016/j.bbr.2015.04.002. Epub 2015 Apr 9. Behav Brain Res. 2015. PMID: 25865152 Free PMC article.
-
Cholinergic Receptor Blockade in the VTA Attenuates Cue-Induced Cocaine-Seeking and Reverses the Anxiogenic Effects of Forced Abstinence.Neuroscience. 2019 Aug 10;413:252-263. doi: 10.1016/j.neuroscience.2019.06.028. Epub 2019 Jul 2. Neuroscience. 2019. PMID: 31271832 Free PMC article.
-
Ventral tegmental area muscarinic receptors modulate depression and anxiety-related behaviors in rats.Neurosci Lett. 2016 Mar 11;616:80-5. doi: 10.1016/j.neulet.2016.01.057. Epub 2016 Jan 29. Neurosci Lett. 2016. PMID: 26828299 Free PMC article.
-
Selective Effects of the Loss of NMDA or mGluR5 Receptors in the Reward System on Adaptive Decision-Making.eNeuro. 2018 Oct 5;5(4):ENEURO.0331-18.2018. doi: 10.1523/ENEURO.0331-18.2018. eCollection 2018 Jul-Aug. eNeuro. 2018. PMID: 30302389 Free PMC article.
References
-
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. - PubMed
-
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature Neuroscience. 2007;10:1020–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

