Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa
- PMID: 23624857
- PMCID: PMC3668082
- DOI: 10.1104/pp.113.216572
Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, quinoa
Abstract
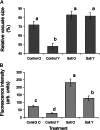
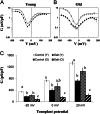
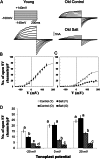
Halophyte species implement a "salt-including" strategy, sequestering significant amounts of Na(+) to cell vacuoles. This requires a reduction of passive Na(+) leak from the vacuole. In this work, we used quinoa (Chenopodium quinoa) to investigate the ability of halophytes to regulate Na(+)-permeable slow-activating (SV) and fast-activating (FV) tonoplast channels, linking it with Na(+) accumulation in mesophyll cells and salt bladders as well as leaf photosynthetic efficiency under salt stress. Our data indicate that young leaves rely on Na(+) exclusion to salt bladders, whereas old ones, possessing far fewer salt bladders, depend almost exclusively on Na(+) sequestration to mesophyll vacuoles. Moreover, although old leaves accumulate more Na(+), this does not compromise their leaf photochemistry. FV and SV channels are slightly more permeable for K(+) than for Na(+), and vacuoles in young leaves express less FV current and with a density unchanged in plants subjected to high (400 mm NaCl) salinity. In old leaves, with an intrinsically lower density of the FV current, FV channel density decreases about 2-fold in plants grown under high salinity. In contrast, intrinsic activity of SV channels in vacuoles from young leaves is unchanged under salt stress. In vacuoles of old leaves, however, it is 2- and 7-fold lower in older compared with young leaves in control- and salt-grown plants, respectively. We conclude that the negative control of SV and FV tonoplast channel activity in old leaves reduces Na(+) leak, thus enabling efficient sequestration of Na(+) to their vacuoles. This enables optimal photosynthetic performance, conferring salinity tolerance in quinoa species.
Figures








Similar articles
-
Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a Halophyte Species, Chenopodium quinoa.Int J Mol Sci. 2013 Apr 29;14(5):9267-85. doi: 10.3390/ijms14059267. Int J Mol Sci. 2013. PMID: 23629664 Free PMC article.
-
Choline but not its derivative betaine blocks slow vacuolar channels in the halophyte Chenopodium quinoa: implications for salinity stress responses.FEBS Lett. 2014 Nov 3;588(21):3918-23. doi: 10.1016/j.febslet.2014.09.003. Epub 2014 Sep 19. FEBS Lett. 2014. PMID: 25240200
-
Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa (Chenopodium quinoa).Physiol Plant. 2012 Sep;146(1):26-38. doi: 10.1111/j.1399-3054.2012.01599.x. Epub 2012 Mar 15. Physiol Plant. 2012. PMID: 22324972
-
The energy cost of the tonoplast futile sodium leak.New Phytol. 2020 Feb;225(3):1105-1110. doi: 10.1111/nph.15758. Epub 2019 Mar 30. New Phytol. 2020. PMID: 30802968 Review.
-
Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops.Ann Bot. 2013 Nov;112(7):1209-21. doi: 10.1093/aob/mct205. Epub 2013 Oct 1. Ann Bot. 2013. PMID: 24085482 Free PMC article. Review.
Cited by
-
A chloroplast retrograde signal, 3'-phosphoadenosine 5'-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination.Elife. 2017 Mar 21;6:e23361. doi: 10.7554/eLife.23361. Elife. 2017. PMID: 28323614 Free PMC article.
-
Developing and validating a high-throughput assay for salinity tissue tolerance in wheat and barley.Planta. 2015 Oct;242(4):847-57. doi: 10.1007/s00425-015-2317-1. Epub 2015 May 20. Planta. 2015. PMID: 25991439
-
Salinity Tolerance of Two Potato Cultivars (Solanum tuberosum) Correlates With Differences in Vacuolar Transport Activity.Front Plant Sci. 2018 Jun 5;9:737. doi: 10.3389/fpls.2018.00737. eCollection 2018. Front Plant Sci. 2018. PMID: 29922314 Free PMC article.
-
Potassium homeostasis and signalling: from the whole plant to the subcellular level.Quant Plant Biol. 2025 May 8;6:e13. doi: 10.1017/qpb.2025.10. eCollection 2025. Quant Plant Biol. 2025. PMID: 40391039 Free PMC article. Review.
-
Plant signaling networks involving Ca(2+) and Rboh/Nox-mediated ROS production under salinity stress.Front Plant Sci. 2015 Jun 10;6:427. doi: 10.3389/fpls.2015.00427. eCollection 2015. Front Plant Sci. 2015. PMID: 26113854 Free PMC article. Review.
References
-
- Adams P, Nelson D, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H. (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138: 171–190 - PubMed
-
- Adams P, Thomas JC, Vernon DM, Bohnert HJ, Jensen RG. (1992) Distinct cellular and organismic responses to salt stress. Plant Cell Physiol 33: 1215–1223
-
- Adolf VI, Shabala S, Andersen MN, Razzaghi F, Jacobsen S-E. (2012) Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil 357: 117–129
-
- Agarie S, Shimoda T, Shimizu Y, Baumann K, Sunagawa H, Kondo A, Ueno O, Nakahara T, Nose A, Cushman JC. (2007) Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J Exp Bot 58: 1957–1967 - PubMed
-
- Allen GJ, Amtmann A, Sanders D. (1998) Calcium-dependent and calcium independent K+ mobilization channels in Vicia faba guard cell vacuoles. J Exp Bot 49: 305–318
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

