Ion channels and regulation of insulin secretion in human β-cells: a computational systems analysis
- PMID: 23624892
- PMCID: PMC3662377
- DOI: 10.4161/isl.24166
Ion channels and regulation of insulin secretion in human β-cells: a computational systems analysis
Abstract
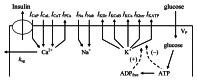
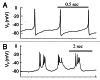
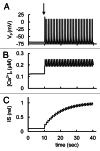
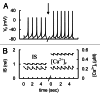
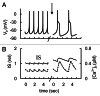
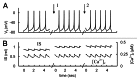
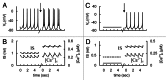
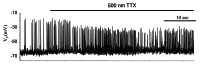
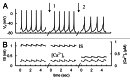
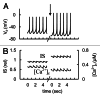
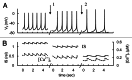
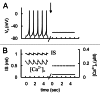
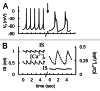
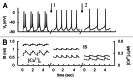
In mammals an increase in glucose leads to block of ATP dependent potassium channels in pancreatic β cells leading to membrane depolarization. This leads to the repetitive firing of action potentials that increases calcium influx and triggers insulin granule exocytosis. Several important differences between species in this process suggest that a dedicated human-oriented approach is advantageous as extrapolating from rodent data may be misleading in several respects. We examined depolarization-induced spike activity in pancreatic human islet-attached β-cells employing whole-cell patch-clamp methods. We also reviewed the literature concerning regulation of insulin secretion by channel activity and constructed a data-based computer model of human β cell function. The model couples the Hodgkin-Huxley-type ionic equations to the equations describing intracellular Ca²⁺ homeostasis and insulin release. On the basis of this model we employed computational simulations to better understand the behavior of action potentials, calcium handling and insulin secretion in human β cells under a wide range of experimental conditions. This computational system approach provides a framework to analyze the mechanisms of human β cell insulin secretion.
Keywords: diabetes; electrophysiology; glucose; mathematical model; membrane potential; ranolazine; tetrodotoxin.
Figures


References
-
- Jacobson DA, Philipson LH. Ion Channels and Insulin secretion. In: Seino S, Bell G, eds. Pancreatic Beta Cell in Health and Desease. Japan: Springer, 2008:91- 110.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
