ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer
- PMID: 23624914
- PMCID: PMC3966979
- DOI: 10.1038/onc.2013.122
ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer
Abstract
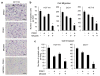
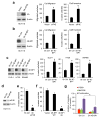
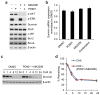
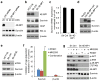
The mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase (MEK/ERK) and phosphatidylinositol 3-kinase (PI3K)/AKT pathways are often concurrently activated by separate genetic alterations in colorectal cancer (CRC), which is associated with CRC progression and poor survival. However, how activating both pathways is required for CRC metastatic progression remains unclear. Our recent study showed that both ERK and AKT signaling are required to activate eukaryotic translation initiation factor 4E (eIF4E)-initiated cap-dependent translation via convergent regulation of the translational repressor 4E-binding protein 1 (4E-BP1) for maintaining CRC transformation. Here, we identified that the activation of cap-dependent translation by cooperative ERK and AKT signaling is critical for promotion of CRC motility and metastasis. In CRC cells with coexistent mutational activation of ERK and AKT pathways, inhibition of either MEK or AKT alone showed limited activity in inhibiting cell migration and invasion, but combined inhibition resulted in profound effects. Genetic blockade of the translation initiation complex by eIF4E knockdown or expression of a dominant active 4E-BP1 mutant effectively inhibited migration, invasion and metastasis of CRC cells, whereas overexpression of eIF4E or knockdown of 4E-BP1 had the opposite effect and markedly reduced their dependence on ERK and AKT signaling for cell motility. Mechanistically, we found that these effects were largely dependent on the increase in mammalian target of rapamycin complex 1 (mTORC1)-mediated survivin translation by ERK and AKT signaling. Despite the modest effect of survivin knockdown on tumor growth, reduction of the translationally regulated survivin profoundly inhibited motility and metastasis of CRC. These findings reveal a critical mechanism underlying the translational regulation of CRC metastatic progression, and suggest that targeting cap-dependent translation may provide a promising treatment strategy for advanced CRC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

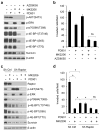
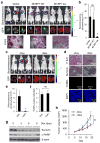
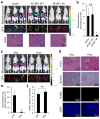
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:1–13. - PubMed
-
- Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. - PubMed
-
- Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR033173/RR/NCRR NIH HHS/United States
- R01 CA175105/CA/NCI NIH HHS/United States
- P20CA150343/CA/NCI NIH HHS/United States
- UL1 RR033173/RR/NCRR NIH HHS/United States
- P20 CA150343/CA/NCI NIH HHS/United States
- P30CA147886/CA/NCI NIH HHS/United States
- KL2 TR000116/TR/NCATS NIH HHS/United States
- P30 CA177558/CA/NCI NIH HHS/United States
- R01CA175105/CA/NCI NIH HHS/United States
- KL2 RR033171/RR/NCRR NIH HHS/United States
- P30 CA147886/CA/NCI NIH HHS/United States
- UL1 TR000117/TR/NCATS NIH HHS/United States
- KL2RR0033171/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

