Latency of Epstein-Barr virus is disrupted by gain-of-function mutant cellular AP-1 proteins that preferentially bind methylated DNA
- PMID: 23625009
- PMCID: PMC3657822
- DOI: 10.1073/pnas.1301577110
Latency of Epstein-Barr virus is disrupted by gain-of-function mutant cellular AP-1 proteins that preferentially bind methylated DNA
Abstract
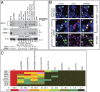
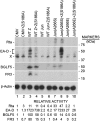
ZEBReplication Activator (ZEBRA), a viral basic zipper protein that initiates the Epstein-Barr viral lytic cycle, binds to DNA and activates transcription through heptamer ZEBRA response elements (ZREs) related to AP-1 sites. A component of the biologic action of ZEBRA is attributable to binding methylated CpGs in ZREs present in the promoters of viral lytic cycle genes. Residue S186 of ZEBRA, Z(S186), which is absolutely required for disruption of latency, participates in the recognition of methylated DNA. We find that mutant cellular AP-1 proteins, Jun(A266S) and Fos(A151S), with alanine-to-serine substitutions homologous to Z(S186), exhibit altered DNA-binding affinity and preferentially bind methylated ZREs. These mutant AP-1 proteins acquire functions of ZEBRA; they activate expression of many viral early lytic cycle gene transcripts in cells harboring latent EBV but are selectively defective in activating expression of some viral proteins and are unable to promote viral DNA replication. Transcriptional activation by mutant c-Jun and c-Fos that have acquired the capacity to bind methylated CpG challenges the paradigm that DNA methylation represses gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A Noncanonical Basic Motif of Epstein-Barr Virus ZEBRA Protein Facilitates Recognition of Methylated DNA, High-Affinity DNA Binding, and Lytic Activation.J Virol. 2019 Jun 28;93(14):e00724-19. doi: 10.1128/JVI.00724-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31068430 Free PMC article.
-
Mutant Cellular AP-1 Proteins Promote Expression of a Subset of Epstein-Barr Virus Late Genes in the Absence of Lytic Viral DNA Replication.J Virol. 2018 Sep 12;92(19):e01062-18. doi: 10.1128/JVI.01062-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021895 Free PMC article.
-
Comparing transcriptional activation and autostimulation by ZEBRA and ZEBRA/c-Fos chimeras.J Virol. 1996 Mar;70(3):1493-504. doi: 10.1128/JVI.70.3.1493-1504.1996. J Virol. 1996. PMID: 8627667 Free PMC article.
-
Amino acid substitutions reveal distinct functions of serine 186 of the ZEBRA protein in activation of early lytic cycle genes and synergy with the Epstein-Barr virus R transactivator.J Virol. 1999 Jun;73(6):4543-51. doi: 10.1128/JVI.73.6.4543-4551.1999. J Virol. 1999. PMID: 10233912 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
Cited by
-
Epstein-Barr virus lytic reactivation regulation and its pathogenic role in carcinogenesis.Int J Biol Sci. 2016 Oct 18;12(11):1309-1318. doi: 10.7150/ijbs.16564. eCollection 2016. Int J Biol Sci. 2016. PMID: 27877083 Free PMC article. Review.
-
Detecting and interpreting DNA methylation marks.Curr Opin Struct Biol. 2018 Dec;53:88-99. doi: 10.1016/j.sbi.2018.06.004. Epub 2018 Jul 19. Curr Opin Struct Biol. 2018. PMID: 30031306 Free PMC article. Review.
-
A Noncanonical Basic Motif of Epstein-Barr Virus ZEBRA Protein Facilitates Recognition of Methylated DNA, High-Affinity DNA Binding, and Lytic Activation.J Virol. 2019 Jun 28;93(14):e00724-19. doi: 10.1128/JVI.00724-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31068430 Free PMC article.
-
5-hydroxymethylation of the EBV genome regulates the latent to lytic switch.Proc Natl Acad Sci U S A. 2015 Dec 29;112(52):E7257-65. doi: 10.1073/pnas.1513432112. Epub 2015 Dec 9. Proc Natl Acad Sci U S A. 2015. PMID: 26663912 Free PMC article.
-
Mutant Cellular AP-1 Proteins Promote Expression of a Subset of Epstein-Barr Virus Late Genes in the Absence of Lytic Viral DNA Replication.J Virol. 2018 Sep 12;92(19):e01062-18. doi: 10.1128/JVI.01062-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021895 Free PMC article.
References
-
- Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: A hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240(4860):1759–1764. - PubMed
-
- Kouzarides T, Packham G, Cook A, Farrell PJ. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene. 1991;6(2):195–204. - PubMed
-
- Petosa C, et al. Structural basis of lytic cycle activation by the Epstein-Barr virus ZEBRA protein. Mol Cell. 2006;21(4):565–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

