Schwann cells seeded in acellular nerve grafts improve functional recovery
- PMID: 23625513
- PMCID: PMC4112584
- DOI: 10.1002/mus.23885
Schwann cells seeded in acellular nerve grafts improve functional recovery
Abstract
Introduction: This study evaluated whether Schwann cells (SCs) from different nerve sources transplanted into cold-preserved acellular nerve grafts (CP-ANGs) would improve functional regeneration compared with nerve isografts.
Methods: SCs isolated and expanded from motor and sensory branches of rat femoral and sciatic nerves were seeded into 14mm CP-ANGs. Growth factor expression, axonal regeneration, and functional recovery were evaluated in a 14-mm rat sciatic injury model and compared with isografts.
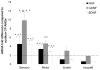
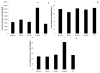
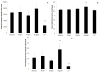
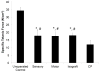
Results: At 14 days, motor or sensory-derived SCs increased expression of growth factors in CP-ANGs versus isografts. After 42 days, histomorphometric analysis found CP-ANGs with SCs and isografts had similar numbers of regenerating nerve fibers. At 84 days, muscle force generation was similar for CP-ANGs with SCs and isografts. SC source did not affect nerve fiber counts or muscle force generation.
Conclusions: SCs transplanted into CP-ANGs increase functional regeneration to isograft levels; however SC nerve source did not have an effect.
Keywords: cell transplantation; growth factor; nerve regeneration; peripheral nerve injury; reinnervation.
Copyright © 2013 Wiley Periodicals, Inc.
Figures


Similar articles
-
Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration.BMC Musculoskelet Disord. 2014 May 21;15:165. doi: 10.1186/1471-2474-15-165. BMC Musculoskelet Disord. 2014. PMID: 24885337 Free PMC article.
-
Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence.Exp Neurol. 2013 Sep;247:165-77. doi: 10.1016/j.expneurol.2013.04.011. Epub 2013 May 3. Exp Neurol. 2013. PMID: 23644284 Free PMC article.
-
Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair.Exp Neurol. 2014 Apr;254:168-79. doi: 10.1016/j.expneurol.2014.01.002. Epub 2014 Jan 15. Exp Neurol. 2014. PMID: 24440805
-
The phenotypic changes of Schwann cells promote the functional repair of nerve injury.Neuropeptides. 2024 Aug;106:102438. doi: 10.1016/j.npep.2024.102438. Epub 2024 May 11. Neuropeptides. 2024. PMID: 38749170 Review.
-
Influences of physical stimulations on the migration and differentiation of Schwann cells involved in peripheral nerve repair.Cell Adh Migr. 2025 Dec;19(1):2450311. doi: 10.1080/19336918.2025.2450311. Epub 2025 Jan 16. Cell Adh Migr. 2025. PMID: 39817348 Free PMC article. Review.
Cited by
-
Engineered Schwann Cell-Based Therapies for Injury Peripheral Nerve Reconstruction.Front Cell Neurosci. 2022 May 6;16:865266. doi: 10.3389/fncel.2022.865266. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35602558 Free PMC article. Review.
-
Gene expression and growth factor analysis in early nerve regeneration following segmental nerve defect reconstruction with a mesenchymal stromal cell-enhanced decellularized nerve allograft.Plast Reconstr Surg Glob Open. 2020 Jan 21;8(1):e2579. doi: 10.1097/GOX.0000000000002579. eCollection 2020 Jan. Plast Reconstr Surg Glob Open. 2020. PMID: 32095395 Free PMC article.
-
Preparation of human decellularized peripheral nerve allograft using amphoteric detergent and nuclease.Neural Regen Res. 2021 Sep;16(9):1890-1896. doi: 10.4103/1673-5374.306091. Neural Regen Res. 2021. PMID: 33510098 Free PMC article.
-
Biological characteristics of tissue engineered-nerve grafts enhancing peripheral nerve regeneration.Stem Cell Res Ther. 2024 Jul 18;15(1):215. doi: 10.1186/s13287-024-03827-9. Stem Cell Res Ther. 2024. PMID: 39020413 Free PMC article.
-
Comparison of acellular nerve allograft modification with Schwann cells or VEGF.Hand (N Y). 2015 Sep;10(3):396-402. doi: 10.1007/s11552-014-9720-0. Hand (N Y). 2015. PMID: 26330769 Free PMC article.
References
-
- Siemionow M, Brzezicki G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. - PubMed
-
- Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurgical focus. 2004;16(5):E1. - PubMed
-
- Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. - PubMed
-
- Bain JR, Mackinnon SE, Hudson AR, Falk RE, Falk JA, Hunter DA, Makino A. Preliminary report of peripheral nerve allografting in primates immunosuppressed with cyclosporin A. Transplant Proc. 1989;21(1 Pt 3):3176–3177. - PubMed
-
- Midha R, Mackinnon SE, Evans PJ, Best TJ, Hare GM, Hunter DA, Falk-Wade JA. Comparison of regeneration across nerve allografts with temporary or continuous cyclosporin A immunosuppression. J Neurosurg. 1993;78(1):90–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

