Emulsified isoflurane postconditioning produces cardioprotection against myocardial ischemia-reperfusion injury in rats
- PMID: 23625523
- PMCID: PMC10717228
- DOI: 10.1007/s12576-013-0261-z
Emulsified isoflurane postconditioning produces cardioprotection against myocardial ischemia-reperfusion injury in rats
Abstract
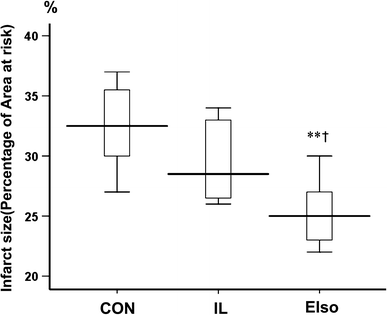
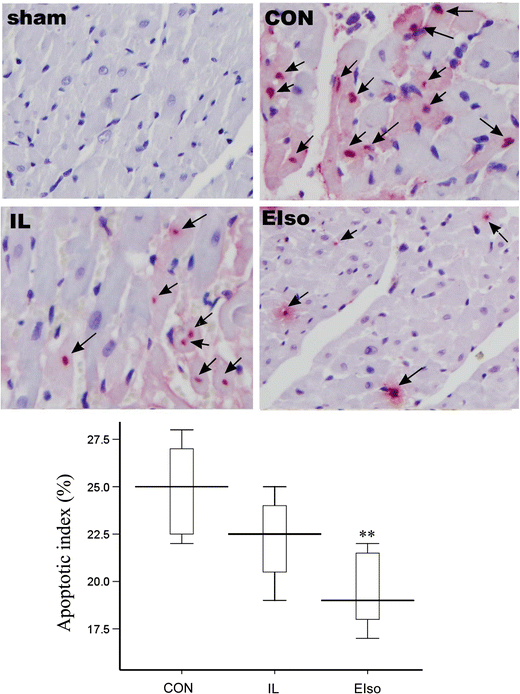
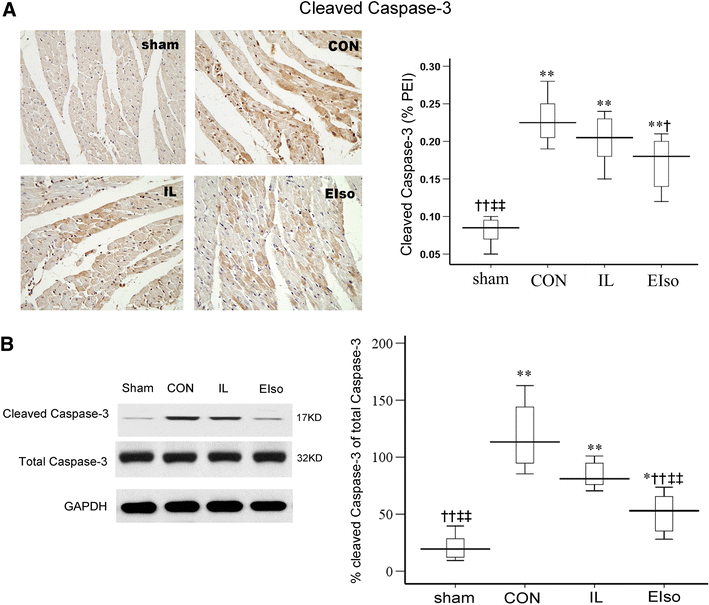
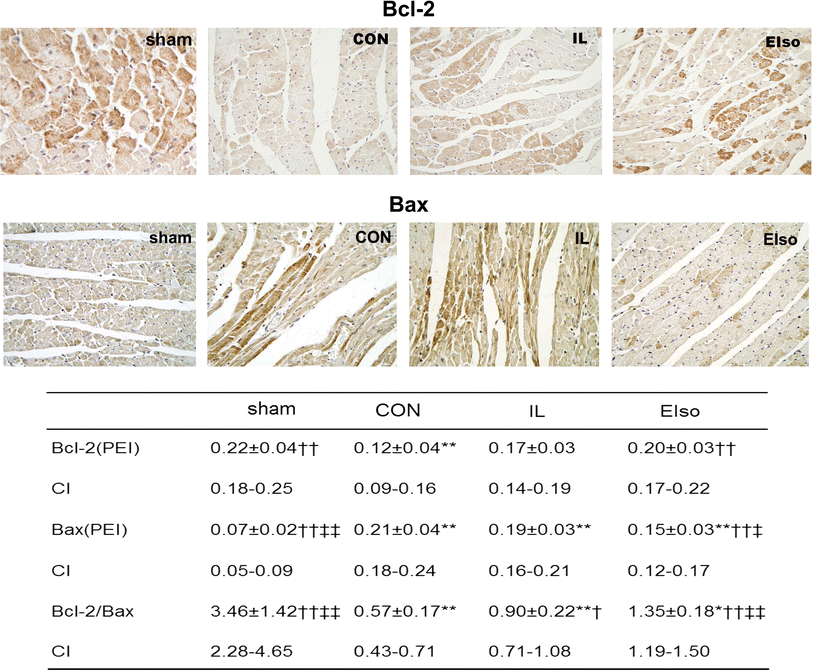
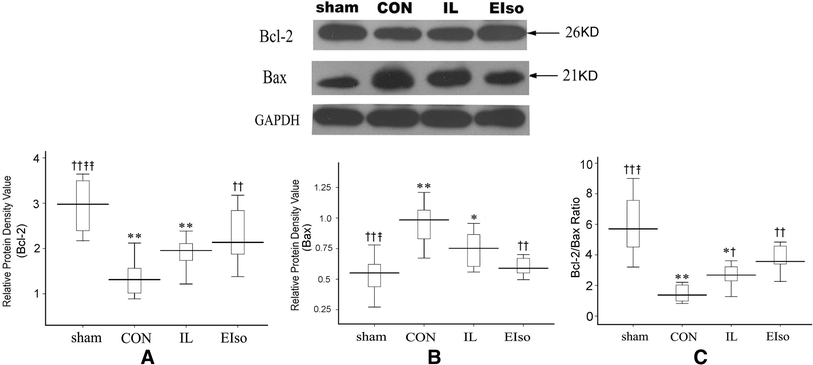
Emulsified isoflurane (EIso) preconditioning can induce cardioprotection. We investigated whether EIso application after ischemia protects hearts against reperfusion injury and whether it is mediated by the inhibition of apoptosis. Rats were subjected to 30-min coronary occlusion followed by 180-min reperfusion. At the onset of reperfusion, rats were intravenously administered saline (sham, control group), 30 % intralipid (IL group) or 2 ml kg(-1) EIso (EIso group) for 30 min. After reperfusion, infarct sizes, myocardial apoptosis and expression of Bcl-2, Bax and caspase-3 proteins were determined. Hemodynamic parameters were not different among groups. Compared with control and intralipid group, EIso limited infarct size, inhibited apoptosis, increased the expression of Bcl-2, decreased the expression of Bax, cleaved caspase-3, and enhanced Bcl-2/Bax ratio. EIso protects hearts against reperfusion injury when administered at the onset of reperfusion, which may be mediated by the inhibition of apoptosis via modulation of the expression of pro- and anti-apoptotic proteins.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

