Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation
- PMID: 23625543
- PMCID: PMC3689287
- DOI: 10.1210/en.2012-2111
Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation
Abstract
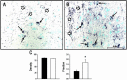
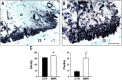
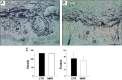
Intrauterine growth restriction (IUGR) is an important fetal developmental problem resulting from 2 broad causes: maternal undernutrition and/or decreased fetal nutrient delivery to the fetus via placental insufficiency. IUGR is often accompanied by up-regulation of the hypothalamo-pituitary-adrenal axis (HPAA). Sheep studies show fetal HPAA autonomy in late gestation. We hypothesized that IUGR, resulting from poor fetal nutrient delivery, up-regulates the fetal baboon HPAA in late gestation, driven by hypothalamo-pituitary glucocorticoid receptor (GR) insensitivity and decreased fetal leptin in peripheral plasma. Maternal baboons were fed as ad libitum controls or nutrient restricted to produce IUGR (fed 70% of the control diet) from 0.16 to 0.9 gestation. Peripheral ACTH, cortisol, and leptin were measured by immunoassays. CRH, arginine vasopressin (AVP), GR, leptin receptor (ObRb), and pro-opiomelanocortin peptide expression were determined immunohistochemically. IUGR fetal peripheral cortisol and ACTH, but not leptin, were increased (P < .05). IUGR increased CRH peptide expression, but not AVP, in the fetal hypothalamic paraventricular nucleus (PVN) and median eminence (P < .05). PVN ObRb peptide expression, but not GR, was decreased (P < .05) with IUGR. ObRb and pro-opiomelanocortin were robustly expressed in the anterior pituitary gland, but ∼1% of cells showed colocalization. We conclude that (1) CRH, not AVP, is the major releasing hormone driving ACTH and cortisol secretion during primate IUGR, (2) fetal HPAA activation was aided by GR insensitivity and decreased ObRb expression in the PVN, and (3) the anterior pituitary is not a site for ObRb effects on the HPAA.
Figures



Comment in
-
Do not turn to the hypothalamus for feedback on stress if you are growth restricted.Endocrinology. 2013 Jul;154(7):2257-9. doi: 10.1210/en.2013-1412. Endocrinology. 2013. PMID: 23794409 No abstract available.
Similar articles
-
Effect of maternal periconceptional undernutrition in sheep on cortisol regulation in offspring from mid-late gestation, through to adulthood.Front Endocrinol (Lausanne). 2023 Feb 2;14:1122432. doi: 10.3389/fendo.2023.1122432. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36817600 Free PMC article.
-
Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum.Ann N Y Acad Sci. 2003 Nov;997:136-49. doi: 10.1196/annals.1290.016. Ann N Y Acad Sci. 2003. PMID: 14644820 Review.
-
Hypothalamic corticotropin-releasing hormone expression in the baboon fetus at mid- and late gestation.Biol Reprod. 1996 Sep;55(3):559-66. doi: 10.1095/biolreprod55.3.559. Biol Reprod. 1996. PMID: 8862772
-
Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance.J Endocrinol. 2013 Apr 29;217(3):275-82. doi: 10.1530/JOE-13-0012. Print 2013 Jun. J Endocrinol. 2013. PMID: 23482706 Free PMC article.
-
Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression.Life Sci. 1998;62(22):1985-98. doi: 10.1016/s0024-3205(98)00027-7. Life Sci. 1998. PMID: 9627097 Review.
Cited by
-
Influence of Low Protein Diet-Induced Fetal Growth Restriction on the Neuroplacental Corticosterone Axis in the Rat.Front Endocrinol (Lausanne). 2019 Mar 11;10:124. doi: 10.3389/fendo.2019.00124. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30915031 Free PMC article.
-
Evidence of lower oxygen reserves during labour in the growth restricted human foetus: a retrospective study.BMC Pregnancy Childbirth. 2017 Jul 1;17(1):209. doi: 10.1186/s12884-017-1392-7. BMC Pregnancy Childbirth. 2017. PMID: 28668074 Free PMC article.
-
Perinatal maternal undernutrition in baboons modulates hepatic mitochondrial function but not metabolites in aging offspring.bioRxiv [Preprint]. 2024 May 5:2024.05.02.592246. doi: 10.1101/2024.05.02.592246. bioRxiv. 2024. PMID: 38746316 Free PMC article. Preprint.
-
Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons.J Physiol. 2017 Jul 1;595(13):4245-4260. doi: 10.1113/JP273928. Epub 2017 May 18. J Physiol. 2017. PMID: 28439937 Free PMC article.
-
The nonhuman primate hypothalamo-pituitary-adrenal axis is an orchestrator of programming-aging interactions: role of nutrition.Nutr Rev. 2020 Dec 1;78(Suppl 2):48-61. doi: 10.1093/nutrit/nuaa018. Nutr Rev. 2020. PMID: 33196092 Free PMC article. Review.
References
-
- Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(suppl 1):4–26 - PubMed
-
- Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72:257–265 - PubMed
-
- Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia. 2012;55:1597–1606 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

