Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa
- PMID: 23625841
- PMCID: PMC3697536
- DOI: 10.1128/JB.02267-12
Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa
Abstract
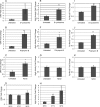
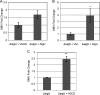
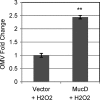
As an opportunistic Gram-negative pathogen, Pseudomonas aeruginosa must be able to adapt and survive changes and stressors in its environment during the course of infection. To aid survival in the hostile host environment, P. aeruginosa has evolved defense mechanisms, including the production of an exopolysaccharide capsule and the secretion of a myriad of degradative proteases and lipases. The production of outer membrane-derived vesicles (OMVs) serves as a secretion mechanism for virulence factors as well as a general bacterial response to envelope-acting stressors. This study investigated the effect of sublethal physiological stressors on OMV production by P. aeruginosa and whether the Pseudomonas quinolone signal (PQS) and the MucD periplasmic protease are critical mechanistic factors in this response. Exposure to some environmental stressors was determined to increase the level of OMV production as well as the activity of AlgU, the sigma factor that controls MucD expression. Overexpression of AlgU was shown to be sufficient to induce OMV production; however, stress-induced OMV production was not dependent on activation of AlgU, since stress caused increased vesiculation in strains lacking algU. We further determined that MucD levels were not an indicator of OMV production under acute stress, and PQS was not required for OMV production under stress or unstressed conditions. Finally, an investigation of the response of P. aeruginosa to oxidative stress revealed that peroxide-induced OMV production requires the presence of B-band but not A-band lipopolysaccharide. Together, these results demonstrate that distinct mechanisms exist for stress-induced OMV production in P. aeruginosa.
Figures



Similar articles
-
Membrane Distribution of the Pseudomonas Quinolone Signal Modulates Outer Membrane Vesicle Production in Pseudomonas aeruginosa.mBio. 2017 Aug 8;8(4):e01034-17. doi: 10.1128/mBio.01034-17. mBio. 2017. PMID: 28790210 Free PMC article.
-
Pseudomonas Quinolone Signal-Induced Outer Membrane Vesicles Enhance Biofilm Dispersion in Pseudomonas aeruginosa.mSphere. 2020 Nov 25;5(6):e01109-20. doi: 10.1128/mSphere.01109-20. mSphere. 2020. PMID: 33239369 Free PMC article.
-
A bilayer-couple model of bacterial outer membrane vesicle biogenesis.mBio. 2012 Mar 13;3(2):e00297-11. doi: 10.1128/mBio.00297-11. Print 2012. mBio. 2012. PMID: 22415005 Free PMC article.
-
Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa.J Bacteriol. 2009 Dec;191(24):7509-19. doi: 10.1128/JB.00722-09. Epub 2009 Oct 16. J Bacteriol. 2009. PMID: 19837799 Free PMC article.
-
Spheres of influence: Porphyromonas gingivalis outer membrane vesicles.Mol Oral Microbiol. 2016 Oct;31(5):365-78. doi: 10.1111/omi.12134. Epub 2015 Oct 23. Mol Oral Microbiol. 2016. PMID: 26466922 Review.
Cited by
-
Environmentally controlled bacterial vesicle-mediated export.Cell Microbiol. 2016 Nov;18(11):1525-1536. doi: 10.1111/cmi.12676. Cell Microbiol. 2016. PMID: 27673272 Free PMC article. Review.
-
The Effect of Growth Stage and Isolation Method on Properties of ClearColi™ Outer Membrane Vesicles (OMVs).Curr Microbiol. 2021 Apr;78(4):1602-1614. doi: 10.1007/s00284-021-02414-y. Epub 2021 Mar 9. Curr Microbiol. 2021. PMID: 33687512
-
Microbial biosynthesis of designer outer membrane vesicles.Curr Opin Biotechnol. 2014 Oct;29:76-84. doi: 10.1016/j.copbio.2014.02.018. Epub 2014 Mar 22. Curr Opin Biotechnol. 2014. PMID: 24667098 Free PMC article. Review.
-
Bacterial membrane vesicles: orchestrators of interkingdom interactions in microbial communities for environmental adaptation and pathogenic dynamics.Front Immunol. 2024 Mar 21;15:1371317. doi: 10.3389/fimmu.2024.1371317. eCollection 2024. Front Immunol. 2024. PMID: 38576623 Free PMC article. Review.
-
Bacterial extracellular vesicles: biotechnological perspective for enhanced productivity.World J Microbiol Biotechnol. 2024 Apr 20;40(6):174. doi: 10.1007/s11274-024-03963-7. World J Microbiol Biotechnol. 2024. PMID: 38642254 Free PMC article. Review.
References
-
- Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

