A putative transmembrane leucine zipper of agrobacterium VirB10 is essential for t-pilus biogenesis but not type IV secretion
- PMID: 23625845
- PMCID: PMC3697533
- DOI: 10.1128/JB.00287-13
A putative transmembrane leucine zipper of agrobacterium VirB10 is essential for t-pilus biogenesis but not type IV secretion
Abstract
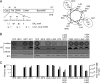
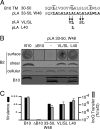
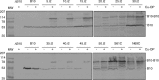
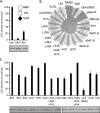
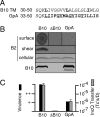
The Agrobacterium tumefaciens VirB/VirD4 type IV secretion system is composed of a translocation channel and an extracellular T pilus. Bitopic VirB10, the VirB7 lipoprotein, and VirB9 interact to form a cell envelope-spanning structural scaffold termed the "core complex" that is required for the assembly of both structures. The related pKM101-encoded core complex is composed of 14 copies each of these VirB homologs, and the transmembrane (TM) α helices of VirB10-like TraF form a 55-Å-diameter ring at the inner membrane. Here, we report that the VirB10 TM helix possesses two types of putative dimerization motifs, a GxxxA (GA4) motif and two leucine (Leu1, Leu2) zippers. Mutations in the Leu1 motif disrupted T-pilus biogenesis, but these or other mutations in the GA4 or Leu2 motif did not abolish substrate transfer. Replacement of the VirB10 TM domain with a nondimerizing poly-Leu/Ala TM domain sequence also blocked pilus production but not substrate transfer or formation of immunoprecipitable complexes with the core subunits VirB7 and VirB9 and the substrate receptor VirD4. The VirB10 TM helix formed weak homodimers in Escherichia coli, as determined with the TOXCAT assay, whereas replacement of the VirB10 TM helix with the strongly dimerizing TM helix from glycophorin A blocked T-pilus biogenesis in A. tumefaciens. Our findings support a model in which VirB10's TM helix contributes to the assembly or activity of the translocation channel as a weakly self-interacting membrane anchor but establishes a heteromeric TM-TM helix interaction via its Leu1 motif that is critical for T-pilus biogenesis.
Figures



Similar articles
-
Agrobacterium VirB10 domain requirements for type IV secretion and T pilus biogenesis.Mol Microbiol. 2009 Feb;71(3):779-94. doi: 10.1111/j.1365-2958.2008.06565.x. Epub 2008 Dec 1. Mol Microbiol. 2009. PMID: 19054325 Free PMC article.
-
Agrobacterium tumefaciens VirB6 protein participates in formation of VirB7 and VirB9 complexes required for type IV secretion.J Bacteriol. 2003 May;185(9):2867-78. doi: 10.1128/JB.185.9.2867-2878.2003. J Bacteriol. 2003. PMID: 12700266 Free PMC article.
-
An Agrobacterium VirB10 mutation conferring a type IV secretion system gating defect.J Bacteriol. 2011 May;193(10):2566-74. doi: 10.1128/JB.00038-11. Epub 2011 Mar 18. J Bacteriol. 2011. PMID: 21421757 Free PMC article.
-
The Agrobacterium VirB/VirD4 T4SS: Mechanism and Architecture Defined Through In Vivo Mutagenesis and Chimeric Systems.Curr Top Microbiol Immunol. 2018;418:233-260. doi: 10.1007/82_2018_94. Curr Top Microbiol Immunol. 2018. PMID: 29808338 Free PMC article. Review.
-
The role of the T-pilus in horizontal gene transfer and tumorigenesis.Curr Opin Microbiol. 2000 Dec;3(6):643-8. doi: 10.1016/s1369-5274(00)00154-5. Curr Opin Microbiol. 2000. PMID: 11121787 Review.
Cited by
-
The transmembrane domain of the Staphylococcus aureus ESAT-6 component EssB mediates interaction with the integral membrane protein EsaA, facilitating partially regulated secretion in a heterologous host.Arch Microbiol. 2018 Sep;200(7):1075-1086. doi: 10.1007/s00203-018-1519-x. Epub 2018 May 8. Arch Microbiol. 2018. PMID: 29737367 Free PMC article.
-
Bacteria-Killing Type IV Secretion Systems.Front Microbiol. 2019 May 21;10:1078. doi: 10.3389/fmicb.2019.01078. eCollection 2019. Front Microbiol. 2019. PMID: 31164878 Free PMC article. Review.
-
A role for FtsA in SPOR-independent localization of the essential Escherichia coli cell division protein FtsN.Mol Microbiol. 2014 Jun;92(6):1212-26. doi: 10.1111/mmi.12623. Epub 2014 May 8. Mol Microbiol. 2014. PMID: 24750258 Free PMC article.
-
The Rickettsia type IV secretion system: unrealized complexity mired by gene family expansion.Pathog Dis. 2016 Aug;74(6):ftw058. doi: 10.1093/femspd/ftw058. Epub 2016 Jun 14. Pathog Dis. 2016. PMID: 27307105 Free PMC article.
-
Inhibiting bacterial secretion systems in the fight against antibiotic resistance.Medchemcomm. 2019 May 8;10(5):682-692. doi: 10.1039/c9md00076c. eCollection 2019 May 1. Medchemcomm. 2019. PMID: 31741728 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

