Inactivation of the Pta-AckA pathway causes cell death in Staphylococcus aureus
- PMID: 23625849
- PMCID: PMC3697545
- DOI: 10.1128/JB.00042-13
Inactivation of the Pta-AckA pathway causes cell death in Staphylococcus aureus
Abstract
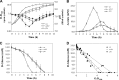
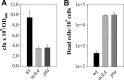
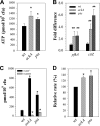
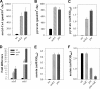
During growth under conditions of glucose and oxygen excess, Staphylococcus aureus predominantly accumulates acetate in the culture medium, suggesting that the phosphotransacetylase-acetate kinase (Pta-AckA) pathway plays a crucial role in bacterial fitness. Previous studies demonstrated that these conditions also induce the S. aureus CidR regulon involved in the control of cell death. Interestingly, the CidR regulon is comprised of only two operons, both encoding pyruvate catabolic enzymes, suggesting an intimate relationship between pyruvate metabolism and cell death. To examine this relationship, we introduced ackA and pta mutations in S. aureus and tested their effects on bacterial growth, carbon and energy metabolism, cid expression, and cell death. Inactivation of the Pta-AckA pathway showed a drastic inhibitory effect on growth and caused accumulation of dead cells in both pta and ackA mutants. Surprisingly, inactivation of the Pta-AckA pathway did not lead to a decrease in the energy status of bacteria, as the intracellular concentrations of ATP, NAD(+), and NADH were higher in the mutants. However, inactivation of this pathway increased the rate of glucose consumption, led to a metabolic block at the pyruvate node, and enhanced carbon flux through both glycolysis and the tricarboxylic acid (TCA) cycle. Intriguingly, disruption of the Pta-AckA pathway also induced the CidR regulon, suggesting that activation of alternative pyruvate catabolic pathways could be an important survival strategy for the mutants. Collectively, the results of this study demonstrate the indispensable role of the Pta-AckA pathway in S. aureus for maintaining energy and metabolic homeostasis during overflow metabolism.
Figures

Similar articles
-
Redox Imbalance Underlies the Fitness Defect Associated with Inactivation of the Pta-AckA Pathway in Staphylococcus aureus.J Proteome Res. 2016 Apr 1;15(4):1205-12. doi: 10.1021/acs.jproteome.5b01089. Epub 2016 Mar 24. J Proteome Res. 2016. PMID: 26975873 Free PMC article.
-
Inactivation of the Pta-AckA pathway impairs fitness of Bacillus anthracis during overflow metabolism.J Bacteriol. 2021 May 1;203(9):e00660-20. doi: 10.1128/JB.00660-20. Epub 2021 Feb 16. J Bacteriol. 2021. PMID: 33593944 Free PMC article.
-
CcpA and CodY Coordinate Acetate Metabolism in Streptococcus mutans.Appl Environ Microbiol. 2017 Mar 17;83(7):e03274-16. doi: 10.1128/AEM.03274-16. Print 2017 Apr 1. Appl Environ Microbiol. 2017. PMID: 28130304 Free PMC article.
-
Amino Acid Catabolism in Staphylococcus aureus and the Function of Carbon Catabolite Repression.mBio. 2017 Feb 14;8(1):e01434-16. doi: 10.1128/mBio.01434-16. mBio. 2017. PMID: 28196956 Free PMC article.
-
Acetate kinase: not just a bacterial enzyme.Trends Microbiol. 2006 Jun;14(6):249-53. doi: 10.1016/j.tim.2006.04.001. Epub 2006 May 4. Trends Microbiol. 2006. PMID: 16678422 Review.
Cited by
-
Characterization of the phosphotransacetylase-acetate kinase pathway for ATP production in Porphyromonas gingivalis.J Oral Microbiol. 2019 Apr 4;11(1):1588086. doi: 10.1080/20002297.2019.1588086. eCollection 2019. J Oral Microbiol. 2019. PMID: 31007866 Free PMC article.
-
Gp05, a Prophage-Encoded Virulence Factor, Contributes to Persistent Methicillin-Resistant Staphylococcus aureus Endovascular Infection.Microbiol Spectr. 2023 Aug 17;11(4):e0060023. doi: 10.1128/spectrum.00600-23. Epub 2023 Jun 26. Microbiol Spectr. 2023. PMID: 37358448 Free PMC article.
-
Quaternized chitosan derivatives inhibit growth and affect biofilm formation of Staphylococcus aureus.Sci Rep. 2025 Aug 12;15(1):29606. doi: 10.1038/s41598-025-11891-1. Sci Rep. 2025. PMID: 40796787 Free PMC article.
-
Acetate Dissimilation and Assimilation in Mycobacterium tuberculosis Depend on Carbon Availability.J Bacteriol. 2015 Oct;197(19):3182-90. doi: 10.1128/JB.00259-15. Epub 2015 Jul 27. J Bacteriol. 2015. PMID: 26216844 Free PMC article.
-
CidR and CcpA Synergistically Regulate Staphylococcus aureus cidABC Expression.J Bacteriol. 2019 Nov 5;201(23):e00371-19. doi: 10.1128/JB.00371-19. Print 2019 Dec 1. J Bacteriol. 2019. PMID: 31501288 Free PMC article.
References
-
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705 - PubMed
-
- Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

