Bifunctional lipocalin ameliorates murine immune complex-induced acute lung injury
- PMID: 23625922
- PMCID: PMC3696655
- DOI: 10.1074/jbc.M112.420331
Bifunctional lipocalin ameliorates murine immune complex-induced acute lung injury
Abstract
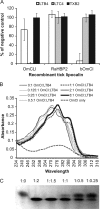
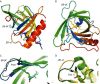
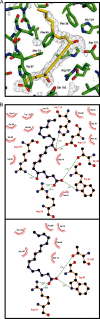
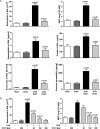
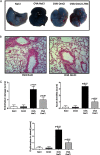
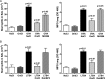
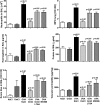
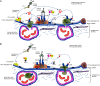
Molecules that simultaneously inhibit independent or co-dependent proinflammatory pathways may have advantages over conventional monotherapeutics. OmCI is a bifunctional protein derived from blood-feeding ticks that specifically prevents complement (C)-mediated C5 activation and also sequesters leukotriene B4 (LTB4) within an internal binding pocket. Here, we examined the effect of LTB4 binding on OmCI structure and function and investigated the relative importance of C-mediated C5 activation and LTB4 in a mouse model of immune complex-induced acute lung injury (IC-ALI). We describe two crystal structures of bacterially expressed OmCI: one binding a C16 fatty acid and the other binding LTB4 (C20). We show that the C5 and LTB4 binding activities of the molecule are independent of each other and that OmCI is a potent inhibitor of experimental IC-ALI, equally dependent on both C5 inhibition and LTB4 binding for full activity. The data highlight the importance of LTB4 in IC-ALI and activation of C5 by the complement pathway C5 convertase rather than by non-C proteases. The findings suggest that dual inhibition of C5 and LTB4 may be useful for treatment of human immune complex-dependent diseases.
Keywords: Acute Lung Injury; Complement; Immune Complex; Immunotherapy; Inflammation; Leukotriene; Lung Injury; Parasite.
Figures



Similar articles
-
Combined inhibition of complement C5 and CD14 markedly attenuates inflammation, thrombogenicity, and hemodynamic changes in porcine sepsis.J Immunol. 2013 Jul 15;191(2):819-27. doi: 10.4049/jimmunol.1201909. Epub 2013 Jun 12. J Immunol. 2013. PMID: 23761634
-
The structure of OMCI, a novel lipocalin inhibitor of the complement system.J Mol Biol. 2007 Jun 8;369(3):784-93. doi: 10.1016/j.jmb.2007.03.064. Epub 2007 Mar 30. J Mol Biol. 2007. PMID: 17445829 Free PMC article.
-
Substance P-regulated leukotriene B4 production promotes acute pancreatitis-associated lung injury through neutrophil reverse migration.Int Immunopharmacol. 2018 Apr;57:147-156. doi: 10.1016/j.intimp.2018.02.017. Epub 2018 Feb 24. Int Immunopharmacol. 2018. PMID: 29482159
-
Role of C3, C5 and anaphylatoxin receptors in acute lung injury and in sepsis.Adv Exp Med Biol. 2012;946:147-59. doi: 10.1007/978-1-4614-0106-3_9. Adv Exp Med Biol. 2012. PMID: 21948367 Free PMC article. Review.
-
The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin.Semin Immunol. 2018 Jun;37:21-29. doi: 10.1016/j.smim.2018.03.002. Epub 2018 Mar 27. Semin Immunol. 2018. PMID: 29602515 Review.
Cited by
-
Allergic eye disease: Blocking LTB4/C5 in vivo suppressed disease and Th2 & Th9 cells.Allergy. 2022 Feb;77(2):660-664. doi: 10.1111/all.15128. Epub 2021 Oct 15. Allergy. 2022. PMID: 34614221 Free PMC article. No abstract available.
-
Structural Characterization and Modeling of a Respiratory Syncytial Virus Fusion Glycoprotein Nanoparticle Vaccine in Solution.Mol Pharm. 2021 Jan 4;18(1):359-376. doi: 10.1021/acs.molpharmaceut.0c00986. Epub 2020 Dec 15. Mol Pharm. 2021. PMID: 33322901 Free PMC article.
-
Structure and function of a family of tick-derived complement inhibitors targeting properdin.Nat Commun. 2022 Jan 14;13(1):317. doi: 10.1038/s41467-021-27920-2. Nat Commun. 2022. PMID: 35031611 Free PMC article.
-
Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury.PLoS One. 2013 May 16;8(5):e64443. doi: 10.1371/journal.pone.0064443. Print 2013. PLoS One. 2013. PMID: 23696894 Free PMC article.
-
A soft tick Ornithodoros moubata salivary protein OmCI is a potent inhibitor to prevent avian complement activation.Ticks Tick Borne Dis. 2020 Mar;11(2):101354. doi: 10.1016/j.ttbdis.2019.101354. Epub 2019 Dec 6. Ticks Tick Borne Dis. 2020. PMID: 31866440 Free PMC article.
References
-
- Lehár J., Krueger A. S., Avery W., Heilbut A. M., Johansen L. M., Price E. R., Rickles R. J., Short G. F., 3rd, Staunton J. E., Jin X., Lee M. S., Zimmermann G. R., Borisy A. A. (2009) Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 27, 659–666 - PMC - PubMed
-
- Hopkins A. L. (2008) Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690 - PubMed
-
- Keith C. T., Borisy A. A., Stockwell B. R. (2005) Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 4, 71–78 - PubMed
-
- Nunn M. A., Sharma A., Paesen G. C., Adamson S., Lissina O., Willis A. C., Nuttall P. A. (2005) Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J. Immunol. 174, 2084–2091 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

