WDR26 functions as a scaffolding protein to promote Gβγ-mediated phospholipase C β2 (PLCβ2) activation in leukocytes
- PMID: 23625927
- PMCID: PMC3675605
- DOI: 10.1074/jbc.M113.462564
WDR26 functions as a scaffolding protein to promote Gβγ-mediated phospholipase C β2 (PLCβ2) activation in leukocytes
Abstract
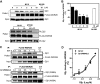
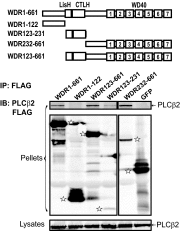
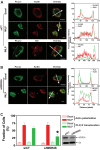
We have recently identified WDR26 as a novel WD40 repeat protein that binds Gβγ and promotes Gβγ signaling during leukocyte migration. Here, we have determined the mechanism by which WDR26 enhances Gβγ-mediated phospholipase C β2 (PLCβ2) activation in leukocytes. We show that WDR26 not only directly bound Gβγ but also PLCβ2. The binding sites of WDR26 and PLCβ2 on Gβ1γ2 were overlapping but not identical. WDR26 used the same domains for binding Gβγ and PLCβ but still formed a signaling complex with Gβγ and PLCβ2 probably due to the fact that WDR26 formed a higher order oligomer through its Lis homology and C-terminal to LisH (LisH-CTLH) and WD40 domains. Additional studies indicated that the formation of higher order oligomers was required for WDR26 to promote PLCβ2 interaction with and activation by Gβγ. Moreover, WDR26 was required for PLCβ2 translocation from the cytosol to the membrane in polarized leukocytes, and the translocation of PLCβ2 was sufficient to cause partial activation of PLCβ2. Collectively, our data indicate that WDR26 functions as a scaffolding protein to promote PLCβ2 membrane translocation and interaction with Gβγ, thereby enhancing PLCβ2 activation in leukocytes. These findings have identified a novel mechanism of regulating Gβγ signaling through a scaffolding protein.
Keywords: Chemotaxis; G Protein βγ; G Protein-coupled Receptors (GPCR); Heterotrimeric G Proteins; Phosphatidylinositol; Phospholipase C; WD40 Repeat Proteins; WDR26.
Figures




References
-
- Oldham W. M., Hamm H. E. (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9, 60–71 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

