γδ T cells are essential effectors of type 1 diabetes in the nonobese diabetic mouse model
- PMID: 23626013
- PMCID: PMC3836168
- DOI: 10.4049/jimmunol.1203502
γδ T cells are essential effectors of type 1 diabetes in the nonobese diabetic mouse model
Abstract
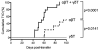
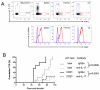
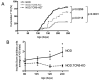
γδ T cells, a lineage of innate-like lymphocytes, are distinguished from conventional αβ T cells in their Ag recognition, cell activation requirements, and effector functions. γδ T cells have been implicated in the pathology of several human autoimmune and inflammatory diseases and their corresponding mouse models, but their specific roles in these diseases have not been elucidated. We report that γδ TCR(+) cells, including both the CD27(-)CD44(hi) and CD27(+)CD44(lo) subsets, infiltrate islets of prediabetic NOD mice. Moreover, NOD CD27(-)CD44(hi) and CD27(+)CD44(lo) γδ T cells were preprogrammed to secrete IL-17, or IFN-γ upon activation. Adoptive transfer of type 1 diabetes (T1D) to T and B lymphocyte-deficient NOD recipients was greatly potentiated when γδ T cells, and specifically the CD27(-) γδ T cell subset, were included compared with transfer of αβ T cells alone. Ab-mediated blockade of IL-17 prevented T1D transfer in this setting. Moreover, introgression of genetic Tcrd deficiency onto the NOD background provided robust T1D protection, supporting a nonredundant, pathogenic role of γδ T cells in this model. The potent contributions of CD27(-) γδ T cells and IL-17 to islet inflammation and diabetes reported in this study suggest that these mechanisms may also underlie human T1D.
Figures


Similar articles
-
Dysregulated B7-1 and B7-2 expression on nonobese diabetic mouse B cells is associated with increased T cell costimulation and the development of insulitis.J Immunol. 2005 Jan 15;174(2):680-7. doi: 10.4049/jimmunol.174.2.680. J Immunol. 2005. PMID: 15634886
-
Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages.Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):E3562-70. doi: 10.1073/pnas.1403424111. Epub 2014 Aug 11. Proc Natl Acad Sci U S A. 2014. PMID: 25114209 Free PMC article.
-
Lineage divergence at the first TCR-dependent checkpoint: preferential γδ and impaired αβ T cell development in nonobese diabetic mice.J Immunol. 2011 Jan 15;186(2):826-37. doi: 10.4049/jimmunol.1002630. Epub 2010 Dec 10. J Immunol. 2011. PMID: 21148803 Free PMC article.
-
Cellular and molecular pathogenic mechanisms of insulin-dependent diabetes mellitus.Ann N Y Acad Sci. 2001 Apr;928:200-11. doi: 10.1111/j.1749-6632.2001.tb05650.x. Ann N Y Acad Sci. 2001. PMID: 11795511 Review.
-
γδTCR-independent origin of neonatal γδ T cells prewired for IL-17 production.Curr Opin Immunol. 2019 Jun;58:60-67. doi: 10.1016/j.coi.2019.04.011. Epub 2019 May 23. Curr Opin Immunol. 2019. PMID: 31128446 Free PMC article. Review.
Cited by
-
The human Stat1 gain-of-function T385M mutation causes expansion of activated T-follicular helper/T-helper 1-like CD4 T cells and sex-biased autoimmunity in specific pathogen-free mice.Front Immunol. 2023 May 19;14:1183273. doi: 10.3389/fimmu.2023.1183273. eCollection 2023. Front Immunol. 2023. PMID: 37275873 Free PMC article.
-
IL-23 drives differentiation of peripheral γδ17 T cells from adult bone marrow-derived precursors.EMBO Rep. 2017 Nov;18(11):1957-1967. doi: 10.15252/embr.201744200. Epub 2017 Aug 30. EMBO Rep. 2017. PMID: 28855306 Free PMC article.
-
Prenatal Betamethasone interferes with immune system development and alters target cells in autoimmune diabetes.Sci Rep. 2019 Feb 4;9(1):1235. doi: 10.1038/s41598-018-37878-9. Sci Rep. 2019. PMID: 30718757 Free PMC article.
-
Mass cytometry reveals the corneal immune cell changes at single cell level in diabetic mice.Front Endocrinol (Lausanne). 2023 Sep 5;14:1253188. doi: 10.3389/fendo.2023.1253188. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37732130 Free PMC article.
-
γδ T Cell-Mediated Immune Responses in Disease and Therapy.Front Immunol. 2014 Nov 10;5:571. doi: 10.3389/fimmu.2014.00571. eCollection 2014. Front Immunol. 2014. PMID: 25426120 Free PMC article. Review.
References
-
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. - PubMed
-
- Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien YH. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. - PubMed
-
- Weintraub BC, Jackson MR, Hedrick SM. Gamma delta T cells can recognize nonclassical MHC in the absence of conventional antigenic peptides. J Immunol. 1994;153:3051–3058. - PubMed
-
- Sciammas R, Bluestone JA. HSV-1 glycoprotein I-reactive TCR gamma delta cells directly recognize the peptide backbone in a conformationally dependent manner. J Immunol. 1998;161:5187–5192. - PubMed
-
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

