Pathology of Takayasu arteritis: A brief review
- PMID: 23626437
- PMCID: PMC3634248
- DOI: 10.4103/0974-2069.107235
Pathology of Takayasu arteritis: A brief review
Abstract
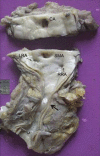
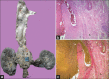
Takayasu arteritis (TA) is a chronic idiopathic and granulomatous vasculitis, manifesting mainly as a panaortitis. Autoimmune cell-mediated immunity is probably responsible for the disease. The inflammation commences from the adventitia and progresses to the intima and leads to, both in adults and children, segmental stenosis, occlusion, dilatation, and/or aneurysm formation. This review focuses briefly on the etiopathogenesis, and describes the pathological and clinical features in adults and children.
Keywords: Large vessel vasculiti; Takayasu arteritis; hypertension.
Conflict of interest statement
Figures







References
-
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. - PubMed
-
- Luqmani R. Large vessel vasculitides: Update for the cardiologist. Curr Opin Cardiol. 2012;27:578–84. - PubMed
-
- Richards BL, March L, Gabriel SE. Epidemiology of large-vessel vasculitides. Best Pract Res Clin Rheumatol. 2010;24:871–3. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

