Advanced hepatocellular carcinoma in adolescence associated with congenital cholestasis: a case description
- PMID: 23626552
- PMCID: PMC3617980
- DOI: 10.1159/000348715
Advanced hepatocellular carcinoma in adolescence associated with congenital cholestasis: a case description
Abstract
This case describes the clinical course and treatment of a 17-year-old male patient with advanced hepatocellular carcinoma (HCC) arising in a non-cirrhotic liver. The disease was thought to be caused by a congenital cholestatic syndrome associated with intermittent oedema in childhood, resembling the rare Aagenaes syndrome. Treatment choices in advanced HCC arising in adolescence are discussed.
Keywords: Adolescence; Chemotherapy; Congenital cholestasis syndrome; Hepatocellular carcinoma; Juvenile; Treatment.
Figures
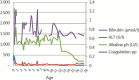
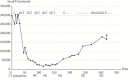
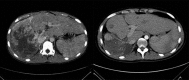
References
-
- Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, Pritchard J, Chapchap P, Keeling J, Plaschkes J, Otte JB, Liver Tumors Study Group of the International Society of Pediatric Oncology Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol. 2002;20:2798–2804. - PubMed
-
- Cruz O, Laguna A, Vancells M, Krauel L, Medina M, Mora J. Fibrolamellar hepatocellular carcinoma in an infant and literature review. J Ped Hematol Oncol. 2008;30:968–971. - PubMed
-
- Drivdal M, Trydal T, Hagve TA, Bergstad I, Aagenaes O. Prognosis, with evaluation of general biochemistry, of liver disease in lymphoedema cholestasis syndrome 1 (LCS1/Aagenaes syndrome) Scand J Gastroenterol. 2006;41:465–471. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

