Perceptual decision making "through the eyes" of a large-scale neural model of v1
- PMID: 23626580
- PMCID: PMC3630335
- DOI: 10.3389/fpsyg.2013.00161
Perceptual decision making "through the eyes" of a large-scale neural model of v1
Abstract
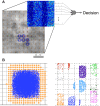
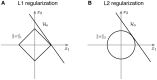
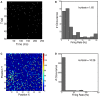
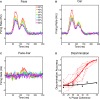
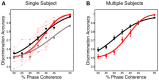
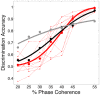
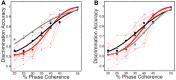
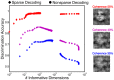
Sparse coding has been posited as an efficient information processing strategy employed by sensory systems, particularly visual cortex. Substantial theoretical and experimental work has focused on the issue of sparse encoding, namely how the early visual system maps the scene into a sparse representation. In this paper we investigate the complementary issue of sparse decoding, for example given activity generated by a realistic mapping of the visual scene to neuronal spike trains, how do downstream neurons best utilize this representation to generate a "decision." Specifically we consider both sparse (L1-regularized) and non-sparse (L2 regularized) linear decoding for mapping the neural dynamics of a large-scale spiking neuron model of primary visual cortex (V1) to a two alternative forced choice (2-AFC) perceptual decision. We show that while both sparse and non-sparse linear decoding yield discrimination results quantitatively consistent with human psychophysics, sparse linear decoding is more efficient in terms of the number of selected informative dimension.
Keywords: computational modeling; decision making; neuronal network; sparse coding.
Figures


Similar articles
-
Mapping visual stimuli to perceptual decisions via sparse decoding of mesoscopic neural activity.Annu Int Conf IEEE Eng Med Biol Soc. 2010;2010:4521. doi: 10.1109/IEMBS.2010.5626062. Annu Int Conf IEEE Eng Med Biol Soc. 2010. PMID: 21095785
-
Predicting Perceptual Decisions Using Visual Cortical Population Responses and Choice History.J Neurosci. 2019 Aug 21;39(34):6714-6727. doi: 10.1523/JNEUROSCI.0035-19.2019. Epub 2019 Jun 24. J Neurosci. 2019. PMID: 31235648 Free PMC article.
-
Feedforward Thalamocortical Connectivity Preserves Stimulus Timing Information in Sensory Pathways.J Neurosci. 2019 Sep 25;39(39):7674-7688. doi: 10.1523/JNEUROSCI.3165-17.2019. Epub 2019 Jul 3. J Neurosci. 2019. PMID: 31270157 Free PMC article.
-
On the neural correlates of visual perception.Cereb Cortex. 1999 Jan-Feb;9(1):4-19. doi: 10.1093/cercor/9.1.4. Cereb Cortex. 1999. PMID: 10022491 Review.
-
Image-Computable Ideal Observers for Tasks with Natural Stimuli.Annu Rev Vis Sci. 2020 Sep 15;6:491-517. doi: 10.1146/annurev-vision-030320-041134. Epub 2020 Jun 24. Annu Rev Vis Sci. 2020. PMID: 32580664 Review.
Cited by
-
Robust Detection of Parkinson's Disease Using Harvested Smartphone Voice Data: A Telemedicine Approach.Telemed J E Health. 2020 Mar;26(3):327-334. doi: 10.1089/tmj.2018.0271. Epub 2019 Apr 26. Telemed J E Health. 2020. PMID: 31033397 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

