Designed ankyrin repeat proteins: a new approach to mimic complex antigens for diagnostic purposes?
- PMID: 23626669
- PMCID: PMC3634029
- DOI: 10.1371/journal.pone.0060688
Designed ankyrin repeat proteins: a new approach to mimic complex antigens for diagnostic purposes?
Abstract
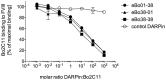
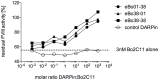
Inhibitory antibodies directed against coagulation factor VIII (FVIII) can be found in patients with acquired and congenital hemophilia A. Such FVIII-inhibiting antibodies are routinely detected by the functional Bethesda Assay. However, this assay has a low sensitivity and shows a high inter-laboratory variability. Another method to detect antibodies recognizing FVIII is ELISA, but this test does not allow the distinction between inhibitory and non-inhibitory antibodies. Therefore, we aimed at replacing the intricate antigen FVIII by Designed Ankyrin Repeat Proteins (DARPins) mimicking the epitopes of FVIII inhibitors. As a model we used the well-described inhibitory human monoclonal anti-FVIII antibody, Bo2C11, for the selection on DARPin libraries. Two DARPins were selected binding to the antigen-binding site of Bo2C11, which mimic thus a functional epitope on FVIII. These DARPins inhibited the binding of the antibody to its antigen and restored FVIII activity as determined in the Bethesda assay. Furthermore, the specific DARPins were able to recognize the target antibody in human plasma and could therefore be used to test for the presence of Bo2C11-like antibodies in a large set of hemophilia A patients. These data suggest, that our approach might be used to isolate epitopes from different sets of anti-FVIII antibodies in order to develop an ELISA-based screening assay allowing the distinction of inhibitory and non-inhibitory anti-FVIII antibodies according to their antibody signatures.
Conflict of interest statement
Figures
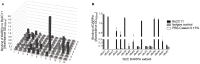

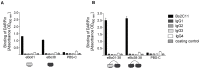

Similar articles
-
Peptide decoys selected by phage display block in vitro and in vivo activity of a human anti-FVIII inhibitor.Blood. 2003 Aug 1;102(3):949-52. doi: 10.1182/blood-2002-06-1886. Epub 2003 Apr 3. Blood. 2003. PMID: 12676786
-
Diagnostic and prognostic value of factor VIII binding antibodies in acquired hemophilia A: data from the GTH-AH 01/2010 study.J Thromb Haemost. 2016 May;14(5):940-7. doi: 10.1111/jth.13304. Epub 2016 Apr 22. J Thromb Haemost. 2016. PMID: 26988717
-
Epitope mapping via selection of anti-FVIII antibody-specific phage-presented peptide ligands that mimic the antibody binding sites.Thromb Haemost. 2015 Feb;113(2):396-405. doi: 10.1160/TH14-01-0101. Epub 2014 Dec 18. Thromb Haemost. 2015. PMID: 25520269
-
B-cell and T-cell epitopes in anti-factor VIII immune responses.Clin Rev Allergy Immunol. 2009 Oct;37(2):80-95. doi: 10.1007/s12016-009-8120-7. Clin Rev Allergy Immunol. 2009. PMID: 19184559 Review.
-
Autoantibodies to factor VIII with catalytic activity.Autoimmun Rev. 2003 Jan;2(1):30-5. doi: 10.1016/s1568-9972(02)00126-x. Autoimmun Rev. 2003. PMID: 12848973 Review.
Cited by
-
Fn3 proteins engineered to recognize tumor biomarker mesothelin internalize upon binding.PLoS One. 2018 May 8;13(5):e0197029. doi: 10.1371/journal.pone.0197029. eCollection 2018. PLoS One. 2018. PMID: 29738555 Free PMC article.
-
The production of the first functional antibody mimetic in higher plants: the chloroplast makes the DARPin G3 for HER2 imaging in oncology.Biol Res. 2022 Oct 23;55(1):32. doi: 10.1186/s40659-022-00400-7. Biol Res. 2022. PMID: 36274167 Free PMC article.
-
Designed Ankyrin Repeat Protein (DARPin) Neutralizers of TcdB from Clostridium difficile Ribotype 027.mSphere. 2019 Oct 2;4(5):e00596-19. doi: 10.1128/mSphere.00596-19. mSphere. 2019. PMID: 31578248 Free PMC article.
-
Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B.PLoS Biol. 2019 Jun 24;17(6):e3000311. doi: 10.1371/journal.pbio.3000311. eCollection 2019 Jun. PLoS Biol. 2019. PMID: 31233493 Free PMC article.
-
High affinity targeting of CD23 inhibits IgE synthesis in human B cells.Immun Inflamm Dis. 2015 Jul 14;3(4):339-49. doi: 10.1002/iid3.72. eCollection 2015 Dec. Immun Inflamm Dis. 2015. PMID: 26732048 Free PMC article.
References
-
- Lenting P, van Mourik J, Mertens K (1998) The life cycle of coagulation factor VIII in view of its structure and function. Blood 92: 3983–3996. - PubMed
-
- Ghosh K, Shetty S (2009) Immune response to FVIII in hemophilia A: an overview of risk factors. Clin Rev Allergy Immunol 37: 58–66. - PubMed
-
- Gringeri A, Mantovani LG, Scalone L, Mannucci PM (2003) Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood 102: 2358–2363. - PubMed
-
- Coutinho A, Kazatchkine MD, Avrameas S (1995) Natural autoantibodies. Curr Opin Immunol 7: 812–818. - PubMed
-
- Irigoyen MB, Primiani L, Felippo M, Candela M, Bianco RP, et al. (2011) A flow cytometry evaluation of anti-FVIII antibodies: correlation with ELISA and Bethesda assay. Haemophilia 17: 267–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

