Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro
- PMID: 23626698
- PMCID: PMC3633995
- DOI: 10.1371/journal.pone.0061540
Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro
Abstract
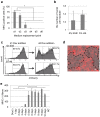
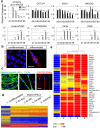
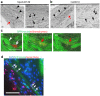
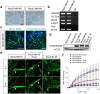
The establishment of human induced pluripotent stem cells (hiPSCs) has enabled the production of in vitro, patient-specific cell models of human disease. In vitro recreation of disease pathology from patient-derived hiPSCs depends on efficient differentiation protocols producing relevant adult cell types. However, myogenic differentiation of hiPSCs has faced obstacles, namely, low efficiency and/or poor reproducibility. Here, we report the rapid, efficient, and reproducible differentiation of hiPSCs into mature myocytes. We demonstrated that inducible expression of myogenic differentiation1 (MYOD1) in immature hiPSCs for at least 5 days drives cells along the myogenic lineage, with efficiencies reaching 70-90%. Myogenic differentiation driven by MYOD1 occurred even in immature, almost completely undifferentiated hiPSCs, without mesodermal transition. Myocytes induced in this manner reach maturity within 2 weeks of differentiation as assessed by marker gene expression and functional properties, including in vitro and in vivo cell fusion and twitching in response to electrical stimulation. Miyoshi Myopathy (MM) is a congenital distal myopathy caused by defective muscle membrane repair due to mutations in DYSFERLIN. Using our induced differentiation technique, we successfully recreated the pathological condition of MM in vitro, demonstrating defective membrane repair in hiPSC-derived myotubes from an MM patient and phenotypic rescue by expression of full-length DYSFERLIN (DYSF). These findings not only facilitate the pathological investigation of MM, but could potentially be applied in modeling of other human muscular diseases by using patient-derived hiPSCs.
Conflict of interest statement
Figures


Similar articles
-
Directed Myogenic Differentiation of Human Induced Pluripotent Stem Cells.Methods Mol Biol. 2016;1353:89-99. doi: 10.1007/7651_2015_257. Methods Mol Biol. 2016. PMID: 25971915
-
Full-length dysferlin expression driven by engineered human dystrophic blood derived CD133+ stem cells.FEBS J. 2013 Dec;280(23):6045-60. doi: 10.1111/febs.12523. Epub 2013 Oct 8. FEBS J. 2013. PMID: 24028392
-
Proteasomal inhibition restores biological function of mis-sense mutated dysferlin in patient-derived muscle cells.J Biol Chem. 2012 Mar 23;287(13):10344-10354. doi: 10.1074/jbc.M111.329078. Epub 2012 Feb 8. J Biol Chem. 2012. Retraction in: J Biol Chem. 2017 Jul 28;292(30):12542. doi: 10.1074/jbc.A111.329078. PMID: 22318734 Free PMC article. Retracted.
-
[Modeling muscular diseases by patient-derived iPS cells].Nihon Yakurigaku Zasshi. 2016 May;147(5):272-6. doi: 10.1254/fpj.147.272. Nihon Yakurigaku Zasshi. 2016. PMID: 27181721 Review. Japanese. No abstract available.
-
Muscle Cells Fix Breaches by Orchestrating a Membrane Repair Ballet.J Neuromuscul Dis. 2018;5(1):21-28. doi: 10.3233/JND-170251. J Neuromuscul Dis. 2018. PMID: 29480214 Free PMC article. Review.
Cited by
-
Human artificial chromosomes for Duchenne muscular dystrophy and beyond: challenges and hopes.Chromosome Res. 2015 Feb;23(1):135-41. doi: 10.1007/s10577-014-9460-6. Chromosome Res. 2015. PMID: 25596829 Review.
-
High-throughput, real-time monitoring of engineered skeletal muscle function using magnetic sensing.J Tissue Eng. 2022 Sep 2;13:20417314221122127. doi: 10.1177/20417314221122127. eCollection 2022 Jan-Dec. J Tissue Eng. 2022. PMID: 36082311 Free PMC article.
-
The Importance of Biophysical and Biochemical Stimuli in Dynamic Skeletal Muscle Models.Front Physiol. 2018 Aug 22;9:1130. doi: 10.3389/fphys.2018.01130. eCollection 2018. Front Physiol. 2018. PMID: 30246791 Free PMC article. Review.
-
Contractile Activity of Myotubes Derived from Human Induced Pluripotent Stem Cells: A Model of Duchenne Muscular Dystrophy.Cells. 2021 Sep 27;10(10):2556. doi: 10.3390/cells10102556. Cells. 2021. PMID: 34685536 Free PMC article.
-
Possible application of muscle specific conditional mouse-derived induced pluripotent stem cells for muscle research.Biochem Biophys Rep. 2020 Feb 1;21:100744. doi: 10.1016/j.bbrep.2020.100744. eCollection 2020 Mar. Biochem Biophys Rep. 2020. PMID: 32025579 Free PMC article.
References
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920. - PubMed
-
- Tiscornia G, Vivas EL, Belmonte JC (2011) Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nature medicine 17: 1570–1576. - PubMed
-
- Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, et al. (2007) Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nature medicine 13: 642–648. - PubMed
-
- Mahmood A, Harkness L, Schroder HD, Abdallah BM, Kassem M (2010) Enhanced differentiation of human embryonic stem cells to mesenchymal progenitors by inhibition of TGF-beta/activin/nodal signaling using SB-431542. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 25: 1216–1233. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

