The calmodulin-binding, short linear motif, NSCaTE is conserved in L-type channel ancestors of vertebrate Cav1.2 and Cav1.3 channels
- PMID: 23626724
- PMCID: PMC3634016
- DOI: 10.1371/journal.pone.0061765
The calmodulin-binding, short linear motif, NSCaTE is conserved in L-type channel ancestors of vertebrate Cav1.2 and Cav1.3 channels
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/7fc1cd8d-1eeb-4ab7-929b-96529cab1c1f
Abstract
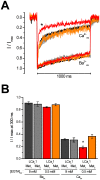
NSCaTE is a short linear motif of (xWxxx(I or L)xxxx), composed of residues with a high helix-forming propensity within a mostly disordered N-terminus that is conserved in L-type calcium channels from protostome invertebrates to humans. NSCaTE is an optional, lower affinity and calcium-sensitive binding site for calmodulin (CaM) which competes for CaM binding with a more ancient, C-terminal IQ domain on L-type channels. CaM bound to N- and C- terminal tails serve as dual detectors to changing intracellular Ca(2+) concentrations, promoting calcium-dependent inactivation of L-type calcium channels. NSCaTE is absent in some arthropod species, and is also lacking in vertebrate L-type isoforms, Cav1.1 and Cav1.4 channels. The pervasiveness of a methionine just downstream from NSCaTE suggests that L-type channels could generate alternative N-termini lacking NSCaTE through the choice of translational start sites. Long N-terminus with an NSCaTE motif in L-type calcium channel homolog LCav1 from pond snail Lymnaea stagnalis has a faster calcium-dependent inactivation than a shortened N-termini lacking NSCaTE. NSCaTE effects are present in low concentrations of internal buffer (0.5 mM EGTA), but disappears in high buffer conditions (10 mM EGTA). Snail and mammalian NSCaTE have an alpha-helical propensity upon binding Ca(2+)-CaM and can saturate both CaM N-terminal and C-terminal domains in the absence of a competing IQ motif. NSCaTE evolved in ancestors of the first animals with internal organs for promoting a more rapid, calcium-sensitive inactivation of L-type channels.
Conflict of interest statement
Figures







References
-
- Chou JJ, Li S, Klee CB, Bax A (2001) Solution structure of Ca(2+)-calmodulin reveals flexible hand-like properties of its domains. Nat Struct Biol 8: 990–997 10.1038/nsb1101–990 [doi];nsb1101–990 [pii]. - PubMed
-
- Yamniuk AP, Vogel HJ (2004) Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol Biotechnol 27: 33–57 MB:27:1: 33 [pii];10.1385/MB:27:1: 33 [doi]. - PubMed
-
- Taylor CW, Laude AJ (2002) IP3 receptors and their regulation by calmodulin and cytosolic Ca2+. Cell Calcium 32: 321–334. S0143416002001859 [pii]. - PubMed
-
- Kiselyov K, Kim JY, Zeng W, Muallem S (2005) Protein-protein interaction and functionTRPC channels. Pflugers Arch 451: 116–124 10.1007/s00424–005–1442–2 [doi]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

