Human S100A9 protein is stabilized by inflammatory stimuli via the formation of proteolytically-resistant homodimers
- PMID: 23626736
- PMCID: PMC3633927
- DOI: 10.1371/journal.pone.0061832
Human S100A9 protein is stabilized by inflammatory stimuli via the formation of proteolytically-resistant homodimers
Abstract
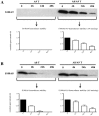
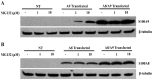
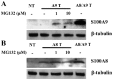
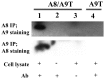
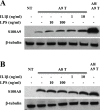
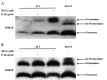
S100A8 and S100A9 are Ca(2+)-binding proteins that are associated with acute and chronic inflammation and cancer. They form predominantly heterodimers even if there are data supporting homodimer formation. We investigated the stability of the heterodimer in myeloid and S100A8/S100A9 over-expressing COS cells. In both cases, S100A8 and S100A9 proteins were not completely degraded even 48 hrs after blocking protein synthesis. In contrast, in single transfected cells, S100A8 protein was completely degraded after 24 h, while S100A9 was completely unstable. However, S100A9 protein expression was rescued upon S100A8 co-expression or inhibition of proteasomal activity. Furthermore, S100A9, but not S100A8, could be stabilized by LPS, IL-1β and TNFα treatment. Interestingly, stimulation of S100A9-transfected COS cells with proteasomal inhibitor or IL-1β lead to the formation of protease resistant S100A9 homodimers. In summary, our data indicated that S100A9 protein is extremely unstable but can be rescued upon co-expression with S100A8 protein or inflammatory stimuli, via proteolytically resistant homodimer formation. The formation of S100A9 homodimers by this mechanism may constitute an amplification step during an inflammatory reaction.
Conflict of interest statement
Figures
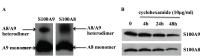
References
-
- Schafer BW, Heizmann CW (1996) The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem. Sci 21: 134–140. - PubMed
-
- Marenholz I, Heizmann CW, Fritz G (2004) S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 322: 1111–1122. - PubMed
-
- Salama I, Malone PS, Mihaimeed F, Jones JL (2008) A review of the S100 proteins in cancer. Eur J Surg Oncol 34: 357–364. - PubMed
-
- Foell D, Frosch M, Sorg C, Roth J (2004) Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta 344: 37–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

