Generating porcine chimeras using inner cell mass cells and parthenogenetic preimplantation embryos
- PMID: 23626746
- PMCID: PMC3633951
- DOI: 10.1371/journal.pone.0061900
Generating porcine chimeras using inner cell mass cells and parthenogenetic preimplantation embryos
Abstract
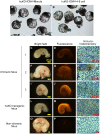
Background: The development and validation of stem cell therapies using induced pluripotent stem (iPS) cells can be optimized through translational research using pigs as large animal models, because pigs have the closest characteristics to humans among non-primate animals. As the recent investigations have been heading for establishment of the human iPS cells with naïve type characteristics, it is an indispensable challenge to develop naïve type porcine iPS cells. The pluripotency of the porcine iPS cells can be evaluated using their abilities to form chimeras. Here, we describe a simple aggregation method using parthenogenetic host embryos that offers a reliable and effective means of determining the chimera formation ability of pluripotent porcine cells. METHODOLOGY/SIGNIFICANT PRINCIPAL FINDINGS: In this study, we show that a high yield of chimeric blastocysts can be achieved by aggregating the inner cell mass (ICM) from porcine blastocysts with parthenogenetic porcine embryos. ICMs cultured with morulae or 4-8 cell-stage parthenogenetic embryos derived from in vitro-matured (IVM) oocytes can aggregate to form chimeric blastocysts that can develop into chimeric fetuses after transfer. The rate of production of chimeric blastocysts after aggregation with host morulae (20/24, 83.3%) was similar to that after the injection of ICMs into morulae (24/29, 82.8%). We also found that 4-8 cell-stage embryos could be used; chimeric blastocysts were produced with a similar efficiency (17/26, 65.4%). After transfer into recipients, these blastocysts yielded chimeric fetuses at frequencies of 36.0% and 13.6%, respectively.
Conclusion/significance: Our findings indicate that the aggregation method using parthenogenetic morulae or 4-8 cell-stage embryos offers a highly reproducible approach for producing chimeric fetuses from porcine pluripotent cells. This method provides a practical and highly accurate system for evaluating pluripotency of undifferentiated cells, such as iPS cells, based on their ability to form chimeras.
Conflict of interest statement
Figures



References
-
- Miki K, Saito A, Uenaka H, Miyagawa S, Shimizu T, et al. (2009) Cardiomyocyte Sheets Derived From Induced Pluripotent Stem (iPS) Cells Improve Cardiac Function and Attenuate Cardiac Remodeling in Myocardial Infarction in Mice. Circulation 120: S721–S721.
-
- Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, et al. (2009) Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett 458: 126–131. - PubMed
-
- Osakada F, Jin Z-B, Hirami Y, Ikeda H, Danjyo T, et al. (2009) In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci 122: 3169–3179. - PubMed
-
- Matsui T, Takano M, Yoshida K, Ono S, Fujisaki C, et al. (2012) Neural stem cells directly differentiated from partially reprogrammed fibroblasts rapidly acquire gliogenic competency. Stem Cells 30: 1109–1119. - PubMed
-
- van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, et al. (2011) Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res 91: 649–658. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

