Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions
- PMID: 23626786
- PMCID: PMC3633878
- DOI: 10.1371/journal.pone.0062187
Lipopolysaccharide disrupts the milk-blood barrier by modulating claudins in mammary alveolar tight junctions
Abstract
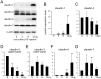
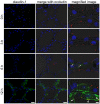
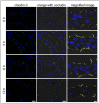
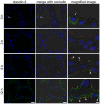
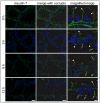
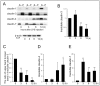
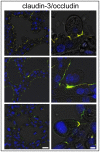
Mastitis, inflammation of the mammary gland, is the most costly common disease in the dairy industry, and is caused by mammary pathogenic bacteria, including Escherichia coli. The bacteria invade the mammary alveolar lumen and disrupt the blood-milk barrier. In normal mammary gland, alveolar epithelial tight junctions (TJs) contribute the blood-milk barrier of alveolar epithelium by blocking the leakage of milk components from the luminal side into the blood serum. In this study, we focused on claudin subtypes that participate in the alveolar epithelial TJs, because the composition of claudins is an important factor that affects TJ permeability. In normal mouse lactating mammary glands, alveolar TJs consist of claudin-3 without claudin-1, -4, and -7. In lipopolysaccharide (LPS)-induced mastitis, alveolar TJs showed 2-staged compositional changes in claudins. First, a qualitative change in claudin-3, presumably caused by phosphorylation and participation of claudin-7 in alveolar TJs, was recognized in parallel with the leakage of fluorescein isothiocyanate-conjugated albumin (FITC-albumin) via the alveolar epithelium. Second, claudin-4 participated in alveolar TJs with claudin-3 and claudin-7 12 h after LPS injection. The partial localization of claudin-1 was also observed by immunostaining. Coinciding with the second change of alveolar TJs, the severe disruption of the blood-milk barrier was recognized by ectopic localization of β-casein and much leakage of FITC-albumin. Furthermore, the localization of toll-like receptor 4 (TLR4) on the luminal side and NFκB activation by LPS was observed in the alveolar epithelial cells. We suggest that the weakening and disruption of the blood-milk barrier are caused by compositional changes of claudins in alveolar epithelial TJs through LPS/TLR4 signaling.
Conflict of interest statement
Figures


References
-
- Akers RM, Nickerson SC (2011) Mastitis and its impact on structure and function in the ruminant mammary gland. J Mammary Gland Biol Neoplasia 16: 275–289. - PubMed
-
- Spencer JP (2008) Management of mastitis in breastfeeding women. Am Fam Physician 78: 727–731. - PubMed
-
- Barlow J (2011) Mastitis therapy and antimicrobial susceptibility: a multispecies review with a focus on antibiotic treatment of mastitis in dairy cattle. J Mammary Gland Biol Neoplasia 16: 383–407. - PubMed
-
- McFadden TB, Akers RM, Capuco AV (1988) Relationship of milk proteins in blood with somatic cell counts in milk of dairy cows. J Dairy Sci 71: 826–834. - PubMed
-
- McFadden TB, Akers RM, Kazmer GW (1987) Alpha-lactalbumin in bovine serum: relationships with udder development and function. J Dairy Sci 70: 259–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

