Crystal structures of a plant trypsin inhibitor from Enterolobium contortisiliquum (EcTI) and of its complex with bovine trypsin
- PMID: 23626794
- PMCID: PMC3633903
- DOI: 10.1371/journal.pone.0062252
Crystal structures of a plant trypsin inhibitor from Enterolobium contortisiliquum (EcTI) and of its complex with bovine trypsin
Abstract
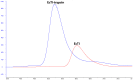
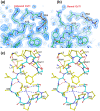
A serine protease inhibitor from Enterolobium contortisiliquum (EcTI) belongs to the Kunitz family of plant inhibitors, common in plant seeds. It was shown that EcTI inhibits the invasion of gastric cancer cells through alterations in integrin-dependent cell signaling pathway. We determined high-resolution crystal structures of free EcTI (at 1.75 Å) and complexed with bovine trypsin (at 2 Å). High quality of the resulting electron density maps and the redundancy of structural information indicated that the sequence of the crystallized isoform contained 176 residues and differed from the one published previously. The structure of the complex confirmed the standard inhibitory mechanism in which the reactive loop of the inhibitor is docked into trypsin active site with the side chains of Arg64 and Ile65 occupying the S1 and S1' pockets, respectively. The overall conformation of the reactive loop undergoes only minor adjustments upon binding to trypsin. Larger deviations are seen in the vicinity of Arg64, driven by the needs to satisfy specificity requirements. A comparison of the EcTI-trypsin complex with the complexes of related Kunitz inhibitors has shown that rigid body rotation of the inhibitors by as much as 15° is required for accurate juxtaposition of the reactive loop with the active site while preserving its conformation. Modeling of the putative complexes of EcTI with several serine proteases and a comparison with equivalent models for other Kunitz inhibitors elucidated the structural basis for the fine differences in their specificity, providing tools that might allow modification of their potency towards the individual enzymes.
Conflict of interest statement
Figures






References
-
- Murzin AG, Chothia C (1992) Protein architecture: new super families. Curr Opin Struct Biol 2: 895–903.
-
- Renko M, Sabotic J, Turk D (2012) Beta trefoil inhibitors from the work of Kunitz onwards. Biol Chem 393: 1043–1054. - PubMed
-
- Horigome D, Satoh H, Itoh N, Mitsunaga K, Oonishi I, et al. (2007) Structural mechanism and photoprotective function of water-soluble chlorophyll-binding protein. J Biol Chem 289: 6525–6531. - PubMed
-
- Cagan RH (1973) Chemostimulatory protein: A new type of taste stimulus. Science 181: 32–35. - PubMed
-
- van Oostrum J, Priestle JP, Grütter MG, Schmitz A (1991) The structure of murine interleukin-1 beta at 2.8 Å resolution. J Struct Biol 107: 189–195. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

