Mouse transplant models for evaluating the oncogenic risk of a self-inactivating XSCID lentiviral vector
- PMID: 23626802
- PMCID: PMC3633865
- DOI: 10.1371/journal.pone.0062333
Mouse transplant models for evaluating the oncogenic risk of a self-inactivating XSCID lentiviral vector
Abstract
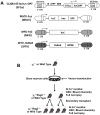
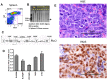
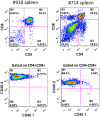
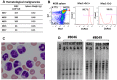
Hematopoietic stem cell gene therapy requires the use of integrating retroviral vectors in order to stably transmit a therapeutic gene to mature blood cells. Human clinical trials have shown that some vector integration events lead to disrupted regulation of proto-oncogenes resulting in disordered hematopoiesis including T-cell leukemia. Newer vectors have been designed to decrease the incidence of these adverse events but require appropriate pre-clinical assays to demonstrate safety. We have used two distinct mouse serial transplant assays to evaluate the safety of a self-inactivating lentiviral vector intended for use in X-linked severe combined immunodeficiency (XSCID) gene therapy trials. These experiments entailed 28 months of total follow-up and included 386 mice. There were no cases in which the XSCID lentiviral vector clearly caused hematopoietic malignancies, although a single case of B cell malignancy was observed that contained the lentiviral vector as a likely passenger event. In contrast, a SFFV-DsRed γ-retroviral vector resulted in clonal transformation events in multiple secondary recipients. Non-specific pathology not related to vector insertions was noted including T cell leukemias arising from irradiated recipient cells. Overall, this comprehensive study of mouse transplant safety assays demonstrate the relative safety of the XSCID lentiviral vector but also highlight the limitations of these assays.
Conflict of interest statement
Figures
Similar articles
-
Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line.Hum Gene Ther Methods. 2012 Oct;23(5):297-308. doi: 10.1089/hgtb.2012.150. Epub 2012 Nov 7. Hum Gene Ther Methods. 2012. PMID: 23075105 Free PMC article.
-
Comparative clonal analysis of reconstitution kinetics after transplantation of hematopoietic stem cells gene marked with a lentiviral SIN or a γ-retroviral LTR vector.Exp Hematol. 2013 Jan;41(1):28-38.e3. doi: 10.1016/j.exphem.2012.09.003. Epub 2012 Sep 16. Exp Hematol. 2013. PMID: 22989760
-
Recombination-activating gene 1 (Rag1)-deficient mice with severe combined immunodeficiency treated with lentiviral gene therapy demonstrate autoimmune Omenn-like syndrome.J Allergy Clin Immunol. 2014 Apr;133(4):1116-23. doi: 10.1016/j.jaci.2013.10.009. Epub 2013 Dec 9. J Allergy Clin Immunol. 2014. PMID: 24332219
-
Gene Therapy for X-Linked Severe Combined Immunodeficiency: Where Do We Stand?Hum Gene Ther. 2016 Feb;27(2):108-16. doi: 10.1089/hum.2015.137. Hum Gene Ther. 2016. PMID: 26790362 Free PMC article. Review.
-
Gene therapy studies in a canine model of X-linked severe combined immunodeficiency.Hum Gene Ther Clin Dev. 2015 Mar;26(1):50-6. doi: 10.1089/humc.2015.004. Epub 2015 Feb 24. Hum Gene Ther Clin Dev. 2015. PMID: 25603151 Free PMC article. Review.
Cited by
-
Genome editing technologies: defining a path to clinic.Mol Ther. 2015 May;23(5):796-806. doi: 10.1038/mt.2015.54. Mol Ther. 2015. PMID: 25943494 Free PMC article. No abstract available.
-
Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency.Sci Transl Med. 2016 Apr 20;8(335):335ra57. doi: 10.1126/scitranslmed.aad8856. Sci Transl Med. 2016. PMID: 27099176 Free PMC article. Clinical Trial.
-
Evaluating the Safety of Retroviral Vectors Based on Insertional Oncogene Activation and Blocked Differentiation in Cultured Thymocytes.Mol Ther. 2016 Jun;24(6):1090-1099. doi: 10.1038/mt.2016.55. Epub 2016 Mar 9. Mol Ther. 2016. PMID: 26957223 Free PMC article.
-
Genomics and epigenetics guided identification of tissue-specific genomic safe harbors.Genome Biol. 2022 Sep 21;23(1):199. doi: 10.1186/s13059-022-02770-3. Genome Biol. 2022. PMID: 36131352 Free PMC article.
-
Safe and Effective Gene Therapy for Murine Wiskott-Aldrich Syndrome Using an Insulated Lentiviral Vector.Mol Ther Methods Clin Dev. 2016 Dec 18;4:1-16. doi: 10.1016/j.omtm.2016.11.001. eCollection 2017 Mar 17. Mol Ther Methods Clin Dev. 2016. PMID: 28344987 Free PMC article.
References
-
- Gaspar HB, Cooray S, Gilmour KC, Parsley KL, Adams S, et al. (2011) Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med 3: 97ra79. - PubMed
-
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, et al. (2009) Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 360: 447–458. - PubMed
-
- Gaspar HB, Cooray S, Gilmour KC, Parsley KL, Zhang F, et al. (2011) Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci Transl Med 3: 97ra80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

