Generation of inducible immortalized dendritic cells with proper immune function in vitro and in vivo
- PMID: 23626840
- PMCID: PMC3633827
- DOI: 10.1371/journal.pone.0062621
Generation of inducible immortalized dendritic cells with proper immune function in vitro and in vivo
Abstract
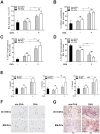
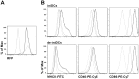
Dendritic cells are the professional antigen presenting cells of innate immunity and key players in maintaining the balance of immune responses. Studies with dendritic cells are mainly limited by their low numbers in vivo and their difficult maintenance in vitro. We differentiated bone marrow cells from transgenic mice expressing an inducible SV40 large T-antigen into dendritic cells. When immortalized by dexamethasone and doxycycline, these cells were stable in long-term culture. In the absence of dexamethasone and doxycycline (de-induction), dendritic cells displayed properties of primary cells, characterized by expression of classical dendritic cell surface markers CD11c, CD11b, MHCII, CD40 and CD86. Furthermore, de-induced lipopolysaccharide activated dendritic cells secreted IL-1β, IL-6, TNFα and IL-12. De-induced, Ovalbumin-loaded dendritic cells polarize CD4(+) T cells into Th1, Th17 and Th2 cells, indicating their correct antigen presenting property. Consistent with intratracheal application of Ovalbumin-loaded primary dendritic cells into mice, the application of de-induced dendritic cells resulted in recruitment of lymphocytes to the lungs. In summary, we successfully expanded dendritic cells using conditional immortalization. The generated dendritic cells demonstrate the characteristic immunophenotype of primary dendritic cells and will facilitate further studies on immunomodulatory properties of dendritic cells.
Conflict of interest statement
Figures
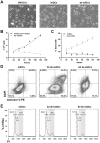
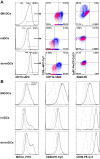

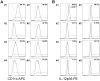
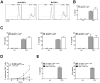
References
-
- Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, et al. (2010) From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur J Immunol 40: 2089–2094. - PubMed
-
- Shortman K, Heath WR (2010) The CD8+ dendritic cell subset. Immunol Rev 234: 18–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

