Stac3 is a novel regulator of skeletal muscle development in mice
- PMID: 23626854
- PMCID: PMC3633831
- DOI: 10.1371/journal.pone.0062760
Stac3 is a novel regulator of skeletal muscle development in mice
Abstract
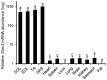
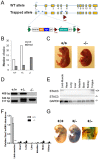
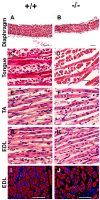
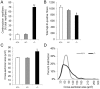
The goal of this study was to identify novel factors that mediate skeletal muscle development or function. We began the study by searching the gene expression databases for genes that have no known functions but are preferentially expressed in skeletal muscle. This search led to the identification of the Src homology three (SH3) domain and cysteine rich (C1) domain 3 (Stac3) gene. We experimentally confirmed that Stac3 mRNA was predominantly expressed in skeletal muscle. We determined if Stac3 plays a role in skeletal muscle development or function by generating Stac3 knockout mice. All Stac3 homozygous mutant mice were found dead at birth, were never seen move, and had a curved body and dropping forelimbs. These mice had marked abnormalities in skeletal muscles throughout the body, including central location of myonuclei, decreased number but increased cross-sectional area of myofibers, decreased number and size of myofibrils, disarrayed myofibrils, and streaming Z-lines. These phenotypes demonstrate that the Stac3 gene plays a critical role in skeletal muscle development and function in mice.
Conflict of interest statement
Figures

Similar articles
-
The SH3 and cysteine-rich domain 3 (Stac3) gene is important to growth, fiber composition, and calcium release from the sarcoplasmic reticulum in postnatal skeletal muscle.Skelet Muscle. 2016 Apr 11;6:17. doi: 10.1186/s13395-016-0088-4. eCollection 2016. Skelet Muscle. 2016. PMID: 27073615 Free PMC article.
-
Identification of the STAC3 gene as a skeletal muscle-specifically expressed gene and a novel regulator of satellite cell differentiation in cattle.J Anim Sci. 2014 Aug;92(8):3284-90. doi: 10.2527/jas.2014-7656. Epub 2014 Jun 19. J Anim Sci. 2014. PMID: 24948655
-
Early life lipid overload in Native American Myopathy is phenocopied by stac3 knockout in zebrafish.Gene. 2025 Feb 5;936:149123. doi: 10.1016/j.gene.2024.149123. Epub 2024 Nov 24. Gene. 2025. PMID: 39592070
-
Cell stress response in skeletal muscle myofibers.Ann N Y Acad Sci. 2006 Jun;1069:472-6. doi: 10.1196/annals.1351.046. Ann N Y Acad Sci. 2006. PMID: 16855175 Review.
-
Structure and function of STAC proteins: Calcium channel modulators and critical components of muscle excitation-contraction coupling.J Biol Chem. 2021 Jul;297(1):100874. doi: 10.1016/j.jbc.2021.100874. Epub 2021 Jun 12. J Biol Chem. 2021. PMID: 34129875 Free PMC article. Review.
Cited by
-
Ca2+ Release Channels Join the 'Resolution Revolution'.Trends Biochem Sci. 2017 Jul;42(7):543-555. doi: 10.1016/j.tibs.2017.04.005. Epub 2017 May 9. Trends Biochem Sci. 2017. PMID: 28499500 Free PMC article. Review.
-
Defective excitation-contraction coupling is partially responsible for impaired contractility in hindlimb muscles of Stac3 knockout mice.Sci Rep. 2016 May 17;6:26194. doi: 10.1038/srep26194. Sci Rep. 2016. PMID: 27184118 Free PMC article.
-
Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):11881-6. doi: 10.1073/pnas.1310571110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818578 Free PMC article.
-
Neurogenetic fetal akinesia and arthrogryposis: genetics, expanding genotype-phenotypes and functional genomics.J Med Genet. 2021 Sep;58(9):609-618. doi: 10.1136/jmedgenet-2020-106901. Epub 2020 Oct 15. J Med Genet. 2021. PMID: 33060286 Free PMC article.
-
Stac Proteins Suppress Ca2+-Dependent Inactivation of Neuronal l-type Ca2+ Channels.J Neurosci. 2018 Oct 24;38(43):9215-9227. doi: 10.1523/JNEUROSCI.0695-18.2018. Epub 2018 Sep 10. J Neurosci. 2018. PMID: 30201773 Free PMC article.
References
-
- Rudnicki MA, Braun T, Hinuma S, Jaenisch R (1992) Inactivation of MyoD in Mice Leads to up-Regulation of the Myogenic HLH Gene Myf-5 and Results in Apparently Normal Muscle Development. Cell 71: 383–390. - PubMed
-
- Wright WE, Sassoon DA, Lin VK (1989) Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56: 607–617. - PubMed
-
- Rhodes SJ, Konieczny SF (1989) Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev 3: 2050–2061. - PubMed
-
- Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiological Reviews 84: 209–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

