Comparative analysis of the peanut witches'-broom phytoplasma genome reveals horizontal transfer of potential mobile units and effectors
- PMID: 23626855
- PMCID: PMC3633829
- DOI: 10.1371/journal.pone.0062770
Comparative analysis of the peanut witches'-broom phytoplasma genome reveals horizontal transfer of potential mobile units and effectors
Abstract
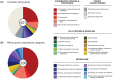
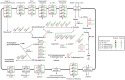
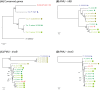
Phytoplasmas are a group of bacteria that are associated with hundreds of plant diseases. Due to their economical importance and the difficulties involved in the experimental study of these obligate pathogens, genome sequencing and comparative analysis have been utilized as powerful tools to understand phytoplasma biology. To date four complete phytoplasma genome sequences have been published. However, these four strains represent limited phylogenetic diversity. In this study, we report the shotgun sequencing and evolutionary analysis of a peanut witches'-broom (PnWB) phytoplasma genome. The availability of this genome provides the first representative of the 16SrII group and substantially improves the taxon sampling to investigate genome evolution. The draft genome assembly contains 13 chromosomal contigs with a total size of 562,473 bp, covering ∼90% of the chromosome. Additionally, a complete plasmid sequence is included. Comparisons among the five available phytoplasma genomes reveal the differentiations in gene content and metabolic capacity. Notably, phylogenetic inferences of the potential mobile units (PMUs) in these genomes indicate that horizontal transfer may have occurred between divergent phytoplasma lineages. Because many effectors are associated with PMUs, the horizontal transfer of these transposon-like elements can contribute to the adaptation and diversification of these pathogens. In summary, the findings from this study highlight the importance of improving taxon sampling when investigating genome evolution. Moreover, the currently available sequences are inadequate to fully characterize the pan-genome of phytoplasmas. Future genome sequencing efforts to expand phylogenetic diversity are essential in improving our understanding of phytoplasma evolution.
Conflict of interest statement
Figures


References
-
- Lee IM, Davis RE, Gundersen-Rindal DE (2000) Phytoplasma: phytopathogenic mollicutes. Annu Rev Microbiol 54: 221–255. - PubMed
-
- IRPCM Phytoplasma/Spiroplasma Working Team – Phytoplasma taxonomy group (2004) ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int J Syst Evol Microbiol 54: 1243–1255. - PubMed
-
- Namba S (2011) Phytoplasmas: A century of pioneering research. J Gen Plant Pathol. 77: 345–349.
-
- Oshima K, Kakizawa S, Nishigawa H, Jung HY, Wei W, et al. (2004) Reductive evolution suggested from the complete genome sequence of a plant-pathogenic phytoplasma. Nat Genet 36: 27–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

