Expression, Purification and Characterization of Three Overlapping Immunodominant Recombinant Fragments from Bordetella pertussis Filamentous Hemagglutinin
- PMID: 23626873
- PMCID: PMC3572703
Expression, Purification and Characterization of Three Overlapping Immunodominant Recombinant Fragments from Bordetella pertussis Filamentous Hemagglutinin
Abstract
Background: Filamentous hemagglutinin (FHA) is one of the most important immunoprotective antigens of Bordetella pertussis (B. pertussis) and a major component of the acellular pertussis vaccine. In the present study, three overlapping recombinant fragments from the immunodominant region of FHA were produced and their immunogenicity was investigated.
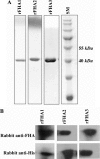
Methods: Three overlapping coding sequences of FHA antigen were amplified from B. pertussis genomic DNA by PCR. Amplified fragments were expressed in Escherichia coli (E. coli) BL21(DE3) strain and purified through His-tag using Nickel-based chromatography. Purified fragments were characterized by SDS-PAGE and Western blotting techniques. In vitro peripheral blood mononuclear cells (PBMC) proliferation and IFN-γ production were assessed in a limited number of healthy adults vaccinated with a commercial acellular pertussis vaccine in response to all purified FHA fragments by H3-Thymidine incorporation and ELISA, respectively.
Results: Recombinant FHA segments were successfully cloned and produced at high levels in E. coli BL21(DE3). SDS-PAGE and Western blot analyses confirmed their purity and reactivity. All three recombinant fragments together with a commercial native FHA were able to induce in vitro PBMC proliferation and IFN-γ production.
Conclusion: Our preliminary results suggest that these overlapping recombinant FHA fragments are immunogenic and may prove to be immuno-protective.
Keywords: Bordetella pertussis; Filamentous hemagglutinin; Immunodominant; Prokaryotic expression; Recombinant antigen.
Figures




Similar articles
-
Restricted antibody response to Bordetella pertussis filamentous hemagglutinin induced by whole-cell and acellular pertussis vaccines.Infect Dis (Lond). 2016 Feb;48(2):127-32. doi: 10.3109/23744235.2015.1093655. Epub 2015 Oct 6. Infect Dis (Lond). 2016. PMID: 26439274
-
High purity and recovery of native filamentous hemagglutinin (FHA) from Bordetella pertussis using affinity chromatography.J Chromatogr B Analyt Technol Biomed Life Sci. 2024 May 15;1239:124122. doi: 10.1016/j.jchromb.2024.124122. Epub 2024 Apr 14. J Chromatogr B Analyt Technol Biomed Life Sci. 2024. PMID: 38669775
-
Interaction of Bordetella pertussis filamentous hemagglutinin with human TLR2: identification of the TLR2-binding domain.APMIS. 2015 Feb;123(2):156-62. doi: 10.1111/apm.12332. Epub 2014 Oct 29. APMIS. 2015. PMID: 25353353
-
Investigation of the Cellular Immune Response to Recombinant Fragments of Filamentous Hemagglutinin and Pertactin of Bordetella pertussis in BALB/c Mice.J Interferon Cytokine Res. 2018 Apr;38(4):161-170. doi: 10.1089/jir.2017.0060. J Interferon Cytokine Res. 2018. PMID: 29638208
-
Bordetella filamentous hemagglutinin and fimbriae: critical adhesins with unrealized vaccine potential.Pathog Dis. 2015 Nov;73(8):ftv079. doi: 10.1093/femspd/ftv079. Epub 2015 Sep 27. Pathog Dis. 2015. PMID: 26416077 Free PMC article. Review.
Cited by
-
Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance.Med Microbiol Immunol. 2018 Feb;207(1):3-26. doi: 10.1007/s00430-017-0524-z. Epub 2017 Nov 21. Med Microbiol Immunol. 2018. PMID: 29164393 Review.
-
Ligation of human Fc receptor like-2 by monoclonal antibodies down-regulates B-cell receptor-mediated signalling.Immunology. 2014 Nov;143(3):341-53. doi: 10.1111/imm.12311. Immunology. 2014. PMID: 24797767 Free PMC article.
-
Immune-Enhancing Effects of Taishan Pinus massoniana Pollen Polysaccharides on DNA Vaccine Expressing Bordetella avium ompA.Front Microbiol. 2016 Feb 2;7:66. doi: 10.3389/fmicb.2016.00066. eCollection 2016. Front Microbiol. 2016. PMID: 26870023 Free PMC article.
References
-
- Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet. 2006;367(9526):1926–1936. - PubMed
-
- Marzouqi I, Richmond P, Fry S, Wetherall J, Muk-kur T. Development of improved vaccines against whooping cough: current status. Hum Vaccin. 2010;6(7):543–553. - PubMed
-
- Berbers GA, de Greeff SC, Mooi FR. Improving pertussis vaccination. Hum Vaccin. 2009;5(7):497–503. - PubMed
-
- Xu Y, Zhang S, Bolgiano B, Tan Y, Asokanathan C, Zhang H, et al. Comparison of recombinant and native pertactin of Bordetella pertussis. Vaccine. 2011;29(10):1974–1980. - PubMed
-
- Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5(5):485–500. - PubMed
LinkOut - more resources
Full Text Sources
