The architecture of Trypanosoma brucei tubulin-binding cofactor B and implications for function
- PMID: 23627368
- PMCID: PMC3806363
- DOI: 10.1111/febs.12308
The architecture of Trypanosoma brucei tubulin-binding cofactor B and implications for function
Abstract
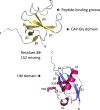
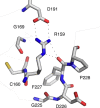
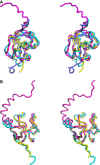
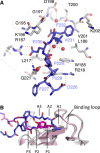
Tubulin-binding cofactor (TBC)-B is implicated in the presentation of α-tubulin ready to polymerize, and at the correct levels to form microtubules. Bioinformatics analyses, including secondary structure prediction, CD, and crystallography, were combined to characterize the molecular architecture of Trypanosoma brucei TBC-B. An efficient recombinant expression system was prepared, material-purified, and characterized by CD. Extensive crystallization screening, allied with the use of limited proteolysis, led to structures of the N-terminal ubiquitin-like and C-terminal cytoskeleton-associated protein with glycine-rich segment domains at 2.35-Å and 1.6-Å resolution, respectively. These are compact globular domains that appear to be linked by a flexible segment. The ubiquitin-like domain contains two lysines that are spatially conserved with residues known to participate in ubiquitinylation, and so may represent a module that, through covalent attachment, regulates the signalling and/or protein degradation associated with the control of microtubule assembly, catastrophe, or function. The TBC-B C-terminal cytoskeleton-associated protein with glycine-rich segment domain, a known tubulin-binding structure, is the only such domain encoded by the T. brucei genome. Interestingly, in the crystal structure, the peptide-binding groove of this domain forms intermolecular contacts with the C-terminus of a symmetry-related molecule, an association that may mimic interactions with the C-terminus of α-tubulin or other physiologically relevant partners. The interaction of TBC-B with the α-tubulin C-terminus may, in particular, protect from post-translational modifications, or simply assist in the shepherding of the protein into polymerization.
Keywords: CAP-Gly domain; CD; crystallography; tubulin-binding; ubiquitin-like.
© 2013 The Authors. FEBS Journal published by John Wiley & Sons Ltd on behalf of FEBS.
Figures
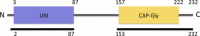


Similar articles
-
Drosophila melanogaster mini spindles TOG3 utilizes unique structural elements to promote domain stability and maintain a TOG1- and TOG2-like tubulin-binding surface.J Biol Chem. 2015 Apr 17;290(16):10149-62. doi: 10.1074/jbc.M114.633826. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720490 Free PMC article.
-
Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition.Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10346-51. doi: 10.1073/pnas.0703876104. Epub 2007 Jun 11. Proc Natl Acad Sci U S A. 2007. PMID: 17563362 Free PMC article.
-
The structure of the complex between α-tubulin, TBCE and TBCB reveals a tubulin dimer dissociation mechanism.J Cell Sci. 2015 May 1;128(9):1824-34. doi: 10.1242/jcs.167387. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908846
-
Solution structure of a ubiquitin-like domain from tubulin-binding cofactor B.J Biol Chem. 2004 Nov 5;279(45):46787-93. doi: 10.1074/jbc.M409422200. Epub 2004 Sep 9. J Biol Chem. 2004. PMID: 15364906
-
Capturing protein tails by CAP-Gly domains.Trends Biochem Sci. 2008 Nov;33(11):535-45. doi: 10.1016/j.tibs.2008.08.006. Epub 2008 Oct 4. Trends Biochem Sci. 2008. PMID: 18835717 Review.
Cited by
-
Crystal structure of the C-terminal domain of tubulin-binding cofactor C from Leishmania major.Mol Biochem Parasitol. 2015 May;201(1):26-30. doi: 10.1016/j.molbiopara.2015.05.003. Epub 2015 May 14. Mol Biochem Parasitol. 2015. PMID: 25982270 Free PMC article.
-
Tubulin cofactors and Arl2 are cage-like chaperones that regulate the soluble αβ-tubulin pool for microtubule dynamics.Elife. 2015 Jul 24;4:e08811. doi: 10.7554/eLife.08811. Elife. 2015. PMID: 26208336 Free PMC article.
-
The structure of tubulin-binding cofactor A from Leishmania major infers a mode of association during the early stages of microtubule assembly.Acta Crystallogr F Struct Biol Commun. 2015 May;71(Pt 5):539-46. doi: 10.1107/S2053230X15000990. Epub 2015 Apr 21. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25945706 Free PMC article.
-
Decreased tubulin-binding cofactor B was involved in the formation disorder of nascent astrocyte processes by regulating microtubule plus-end growth through binding with end-binding proteins 1 and 3 after chronic alcohol exposure.Front Cell Neurosci. 2022 Oct 25;16:989945. doi: 10.3389/fncel.2022.989945. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36385945 Free PMC article.
References
-
- Szymanski D. Tubulin folding cofactors: half a dozen for a dimer. Curr Biol. 2002;12:R767–R769. - PubMed
-
- Lundin VF, Leroux MR, Stirling PC. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010;35:288–297. - PubMed
-
- Vainberg IE, Lewis SA, Rommelaere H, Ampe C, Vandekerckhove J, Klein HL, Cowan NJ. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–873. - PubMed
-
- Muñoz IG, Yébenes H, Zhou M, Mesa P, Serna M, Park AY, Bragado-Nilsson E, Beloso A, de Cárcer G, Malumbres M, et al. Crystal structure of the open conformation of the mammalian chaperonin CCT in complex with tubulin. Nat Struct Mol Biol. 2011;18:14–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

