A Lectin-EGF antibody promotes regulatory T cells and attenuates nephrotoxic nephritis via DC-SIGN on dendritic cells
- PMID: 23627732
- PMCID: PMC3651349
- DOI: 10.1186/1479-5876-11-103
A Lectin-EGF antibody promotes regulatory T cells and attenuates nephrotoxic nephritis via DC-SIGN on dendritic cells
Abstract
Background: Interactions between dendritic cells (DCs) and T cells play a critical role in the development of glomerulonephritis, which is a common cause of chronic kidney disease. DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), an immune-regulating molecule of the C-type lectin family, is mainly expressed on DCs and mediates DC adhesion and migration, inflammation, activation of primary T cells. DC-SIGN triggers immune responses and is involved in the immune escape of pathogens and tumours. In addition, ligation of DC-SIGN on DCs actively primes DCs to induce Tregs. Under certain conditions, DC-SIGN signalling may result in inhibition of DC maturation, by promoting regulatory T cell (Treg) function and affecting Th1/Th2 bias.
Methods: A rat model of nephrotoxic nephritis was used to investigate the therapeutic effects of an anti-lectin-epidermal growth factor (EGF) antibody on glomerulonephritis. DCs were induced by human peripheral blood mononuclear cells in vitro. The expression of DC surface antigens were detected using flow cytometry; the levels of cytokines were detected by ELISA and qPCR, respectively; the capability of DCs to stimulate T cell proliferation was examined by mixed lymphocyte reaction; PsL-EGFmAb targeting to DC-SIGN on DCs was identified by immunoprecipitation.
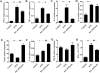
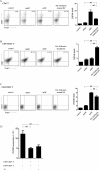
Results: Anti-Lectin-EGF antibody significantly reduced global crescent formation, tubulointerstitial injury and improved renal function impairment through inhibiting DC maturation and modulating Foxp3 expression and the Th1/Th2 cytokine balance in kidney. Binding of anti-Lectin-EGF antibody to DC-SIGN on human DCs inhibited DC maturation, increased IL-10 production from DCs and enhanced CD4+CD25+ Treg functions.
Conclusions: Our results suggest that treatment with anti-Lectin-EGF antibody modulates DCs to suppressive DCs and enhances Treg functions, contributing to the attenuation of renal injury in a rat model of nephrotoxic nephritis.
Figures





Similar articles
-
DC-SIGN modulates DC maturation and function in rat renal tubulointerstitial lesions.Front Biosci (Landmark Ed). 2012 Jan 1;17(5):1795-803. doi: 10.2741/4019. Front Biosci (Landmark Ed). 2012. PMID: 22201836
-
Enterocyte dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin expression in inflammatory bowel disease.World J Gastroenterol. 2015 Jan 7;21(1):187-95. doi: 10.3748/wjg.v21.i1.187. World J Gastroenterol. 2015. PMID: 25574091 Free PMC article.
-
Overexpression of Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Nonintegrin in Dendritic Cells Protecting against Aspergillosis.Chin Med J (Engl). 2018 Nov 5;131(21):2575-2582. doi: 10.4103/0366-6999.244103. Chin Med J (Engl). 2018. PMID: 30381591 Free PMC article.
-
C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity.Cell Signal. 2010 Oct;22(10):1397-405. doi: 10.1016/j.cellsig.2010.03.018. Epub 2010 Apr 2. Cell Signal. 2010. PMID: 20363321 Free PMC article. Review.
-
C-type lectins on dendritic cells: key modulators for the induction of immune responses.Biochem Soc Trans. 2008 Dec;36(Pt 6):1478-81. doi: 10.1042/BST0361478. Biochem Soc Trans. 2008. PMID: 19021579 Review.
Cited by
-
Update on crescentic glomerulonephritis.Semin Immunopathol. 2014 Jul;36(4):479-90. doi: 10.1007/s00281-014-0435-7. Epub 2014 Jun 20. Semin Immunopathol. 2014. PMID: 24948005 Review.
-
Targeting C-Type Lectin Receptors for Cancer Immunity.Front Immunol. 2015 Aug 24;6:408. doi: 10.3389/fimmu.2015.00408. eCollection 2015. Front Immunol. 2015. PMID: 26379663 Free PMC article. Review.
-
Role of Dendritic Cells in the Induction of Lymphocyte Tolerance.Front Immunol. 2015 Oct 20;6:535. doi: 10.3389/fimmu.2015.00535. eCollection 2015. Front Immunol. 2015. PMID: 26539197 Free PMC article. Review.
-
Profiling dendritic cells subsets in renal tissue of patients with crescentic glomerulonephritis.Int Urol Nephrol. 2025 Jan;57(1):263-273. doi: 10.1007/s11255-024-04175-6. Epub 2024 Jul 29. Int Urol Nephrol. 2025. PMID: 39069601
-
Vitamin C Attenuates Hemorrhagic Shock-induced Dendritic Cell-specific Intercellular Adhesion Molecule 3-grabbing Nonintegrin Expression in Tubular Epithelial Cells and Renal Injury in Rats.Chin Med J (Engl). 2016 Jul 20;129(14):1731-6. doi: 10.4103/0366-6999.185868. Chin Med J (Engl). 2016. PMID: 27411463 Free PMC article.
References
-
- Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005;68:S82–S86. - PubMed
-
- Kitching AR, Holdsworth SR, Tipping PG. Crescentic glomerulonephritis–a manifestation of a nephritogenic Th1 response? Histol Histopathol. 2000;15:993–1003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

