Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture
- PMID: 23628342
- PMCID: PMC3653780
- DOI: 10.1186/1754-6834-6-59
Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture
Abstract
Background: Lignocellulosic ethanol is a viable alternative to petroleum-based fuels with the added benefit of potentially lower greenhouse gas emissions. Consolidated bioprocessing (simultaneous enzyme production, hydrolysis and fermentation; CBP) is thought to be a low-cost processing scheme for lignocellulosic ethanol production. However, no single organism has been developed which is capable of high productivity, yield and titer ethanol production directly from lignocellulose. Consortia of cellulolytic and ethanologenic organisms could be an attractive alternate to the typical single organism approaches but implementation of consortia has a number of challenges (e.g., control, stability, productivity).
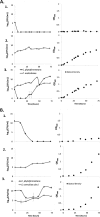
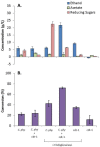
Results: Ethanol is produced from α-cellulose using a consortium of C. phytofermentans and yeast that is maintained by controlled oxygen transport. Both Saccharomyces cerevisiae cdt-1 and Candida molischiana "protect" C. phytofermentans from introduced oxygen in return for soluble sugars released by C. phytofermentans hydrolysis. Only co-cultures were able to degrade filter paper when mono- and co-cultures were incubated at 30°C under semi-aerobic conditions. Using controlled oxygen delivery by diffusion through neoprene tubing at a calculated rate of approximately 8 μmol/L hour, we demonstrate establishment of the symbiotic relationship between C. phytofermentans and S. cerevisiae cdt-1 and maintenance of populations of 105 to 106 CFU/mL for 50 days. Comparable symbiotic population dynamics were observed in scaled up 500 mL bioreactors as those in 50 mL shake cultures. The conversion of α-cellulose to ethanol was shown to improve with additional cellulase indicating a limitation in hydrolysis rate. A co-culture of C. phytofermentans and S. cerevisiae cdt-1 with added endoglucanase produced approximately 22 g/L ethanol from 100 g/L α-cellulose compared to C. phytofermentans and S. cerevisiae cdt-1 mono-cultures which produced approximately 6 and 9 g/L, respectively.
Conclusion: This work represents a significant step toward developing consortia-based bioprocessing systems for lignocellulosic biofuels production which utilize scalable, environmentally-mediated symbiosis mechanisms to provide consortium stability.
Figures




References
-
- Shong J. Jimenez Diaz MR, Collins CH: Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotech. 2012;2:1–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

