Primary care provider practices and beliefs related to cervical cancer screening with the HPV test in Federally Qualified Health Centers
- PMID: 23628517
- PMCID: PMC4547778
- DOI: 10.1016/j.ypmed.2013.04.012
Primary care provider practices and beliefs related to cervical cancer screening with the HPV test in Federally Qualified Health Centers
Abstract
Objective: Cervical cancer screening using the human papillomavirus (HPV) test and Pap test together (co-testing) is an option for average-risk women ≥ 30 years of age. With normal co-test results, screening intervals can be extended. The study objective is to assess primary care provider practices, beliefs, facilitators and barriers to using the co-test and extending screening intervals among low-income women.
Method: Data were collected from 98 providers in 15 Federally Qualified Health Center (FQHC) clinics in Illinois between August 2009 and March 2010 using a cross-sectional survey.
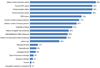
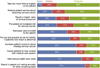
Results: 39% of providers reported using the co-test, and 25% would recommend a three-year screening interval for women with normal co-test results. Providers perceived greater encouragement for co-testing than for extending screening intervals with a normal co-test result. Barriers to extending screening intervals included concerns about patients not returning annually for other screening tests (77%), patient concerns about missing cancer (62%), and liability (52%).
Conclusion: Among FQHC providers in Illinois, few administered the co-test for screening and recommended appropriate intervals, possibly due to concerns over loss to follow-up and liability. Education regarding harms of too-frequent screening and false positives may be necessary to balance barriers to extending screening intervals.
Keywords: Cervical cancer screening; HPV testing; Screening guidelines.
Published by Elsevier Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interests.
Figures
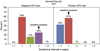

Comment in
-
Prevention of human papillomavirus-related diseases: Impediments to progress.Prev Med. 2013 Nov;57(5):407-8. doi: 10.1016/j.ypmed.2013.08.005. Epub 2013 Aug 14. Prev Med. 2013. PMID: 23954187 No abstract available.
References
-
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 109: cervical cytology screening. Obstet. Gynecol. 2009;114(6):1409–1420. - PubMed
-
- ACOG Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet. Gynecol. 2012;120(5):1222–1238. - PubMed
-
- Ahmad F, Stewart DE, Cameron JI, Hyman I. Rural physicians' perspectives on cervical and breast cancer screening: a gender-based analysis. J. Womens Health Gend. Based Med. 2001;10(2):201–208. - PubMed
-
- Apgar B, Kittendorf AL, Bettcher CM, Wong J, Kaufman AJ. Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am. Fam. Physician. 2009;80(2):147–155. - PubMed
-
- Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(10 Suppl.):2910–2918. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

