Configurational assignments of conformationally restricted bis-monoterpene hydroquinones: utility in exploration of endangered plants
- PMID: 23628705
- PMCID: PMC4139972
- DOI: 10.1016/j.bbagen.2013.04.029
Configurational assignments of conformationally restricted bis-monoterpene hydroquinones: utility in exploration of endangered plants
Abstract
Background: Endangered plant species are an important resource for new chemistry. Lindera melissifolia is native to the Southeastern U.S. and scarcely populates the edges of lakes and ponds. Quantum mechanics (QM) used in combination with NMR/ECD is a powerful tool for the assignment of absolute configuration in lieu of X-ray crystallography.
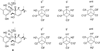
Methods: The EtOAc extract of L. melissifolia was subject to chromatographic analysis by VLC and HPLC. Spin-spin coupling constant (SSCC) were calculated using DFT at the MPW1PW91/6-31G(d,p) level for all staggered rotamers. ECD calculations employed Amber* force fields followed by PM6 semi-empirical optimizations. Hetero- and homo-nuclear coupling constants were extracted from 1D (1)H, E.COSY and HETLOC experiments.
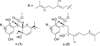
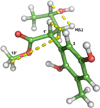
Results: Two meroterpenoids, melissifolianes A (1) and B (2) were purified and their 2-D structures elucidated using NMR and HRESIMS. The relative configuration of 1 was established using the combination of NOE-based distance restraints and the comparisons of experimental and calculated SSCCs. The comparison of calculated and experimental ECD assigned the absolute configuration of 1. The relative configuration of a racemic mixture, melissifoliane B (2) was established utilizing J-based analysis combined with QM and NMR techniques.Conclusion Our study of the Lindera melissifolia metabolome exemplifies how new chemistry remains undiscovered among the numerous endangered plant species and demonstrates how analysis by ECD and NMR combined with various QM calculations is a sensible approach to support the stereochemical assignment of molecules with conformationally restricted conformations.
General significance: QM-NMR/ECD combined approaches are of utility for unambiguous assignment of 3-D structures, especially with limited plant material and when a molecule is conformationally restricted. Conservation of an endangered plant species can be supported through identification of its new chemistry and utilization of that chemistry for commercial purposes.
Copyright © 2013. Published by Elsevier B.V.
Figures



References
-
- Pitman NCA, Jørgensen PM. Estimating the size of the world's threatened flora. Science. 2002;298:989. - PubMed
-
- USDA. [accessed 4/10/2011]; http://plants.usda.gov/threat.html.
-
- Meng Y, Krzysiak AJ, Durako MJ, Kunzelman JI, Wright JLC. Flavones and flavone glycosides from Halophila johnsonii. Phytochemistry. 2008;69:2603–2608. - PubMed
-
- Binns SE, Hudson J, Merali S, Arnason JT. Antiviral activity of characterized extracts from echinacea spp. (Heliantheae: Asteraceae) against virus (HSV-I) Planta Med. 2002;68:780–783. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

