Structure, function, and biology of the Enterococcus faecalis cytolysin
- PMID: 23628786
- PMCID: PMC3709268
- DOI: 10.3390/toxins5050895
Structure, function, and biology of the Enterococcus faecalis cytolysin
Abstract
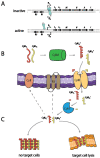
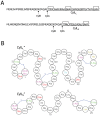
Enterococcus faecalis is a Gram-positive commensal member of the gut microbiota of a wide range of organisms. With the advent of antibiotic therapy, it has emerged as a multidrug resistant, hospital-acquired pathogen. Highly virulent strains of E. faecalis express a pore-forming exotoxin, called cytolysin, which lyses both bacterial and eukaryotic cells in response to quorum signals. Originally described in the 1930s, the cytolysin is a member of a large class of lanthionine-containing bacteriocins produced by Gram-positive bacteria. While the cytolysin shares some core features with other lantibiotics, it possesses unique characteristics as well. The current understanding of cytolysin biosynthesis, structure/function relationships, and contribution to the biology of E. faecalis are reviewed, and opportunities for using emerging technologies to advance this understanding are discussed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

