NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors
- PMID: 23628805
- PMCID: PMC3700668
- DOI: 10.1038/icb.2013.17
NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors
Abstract
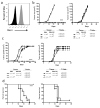
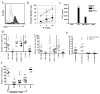
Tumor heterogeneity presents a substantial barrier to increasing clinical responses mediated by targeted therapies. Broadening the immune response elicited by treatments that target a single antigen is necessary for the elimination of tumor variants that fail to express the targeted antigen. In this study, it is shown that adoptive transfer of T cells bearing a chimeric antigen receptor (CAR) inhibited the growth of target-expressing and -deficient tumor cells within ovarian and lymphoma tumors. Mice bearing the ID8 ovarian or RMA lymphoma tumors were treated with T cells transduced with a NKG2D-based CAR (chNKG2D). NKG2D CAR T-cell therapy protected mice from heterogeneous RMA tumors. Moreover, adoptive transfer of chNKG2D T cells mediated tumor protection against highly heterogeneous ovarian tumors in which 50, 20 or only 7% of tumor cells expressed significant amounts of NKG2D ligands. CAR T cells did not mediate an in vivo response against tumor cells that did not express sufficient amounts of NKG2D ligands, and the number of ligand-expressing tumor cells correlated with therapeutic efficacy. In addition, tumor-free surviving mice were protected against a tumor re-challenge with NKG2D ligand-negative ovarian tumor cells. These data indicate that NKG2D CAR T-cell treatment can be an effective therapy against heterogeneous tumors and induce tumor-specific immunity against ligand-deficient tumor cells.
Conflict of interest statement
Figures

Similar articles
-
Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer.Cancer Res. 2007 May 15;67(10):5003-8. doi: 10.1158/0008-5472.CAN-06-4047. Cancer Res. 2007. PMID: 17510432
-
Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways.Cancer Res. 2007 Nov 15;67(22):11029-36. doi: 10.1158/0008-5472.CAN-07-2251. Cancer Res. 2007. PMID: 18006849
-
Augmented anti-tumor activity of NK-92 cells expressing chimeric receptors of TGF-βR II and NKG2D.Cancer Immunol Immunother. 2017 Apr;66(4):537-548. doi: 10.1007/s00262-017-1959-1. Epub 2017 Feb 9. Cancer Immunol Immunother. 2017. PMID: 28184969 Free PMC article.
-
NKG2D CARs as cell therapy for cancer.Cancer J. 2014 Mar-Apr;20(2):156-9. doi: 10.1097/PPO.0000000000000029. Cancer J. 2014. PMID: 24667963 Free PMC article. Review.
-
Fusion Proteins of NKG2D/NKG2DL in Cancer Immunotherapy.Int J Mol Sci. 2018 Jan 7;19(1):177. doi: 10.3390/ijms19010177. Int J Mol Sci. 2018. PMID: 29316666 Free PMC article. Review.
Cited by
-
How Chimeric Antigen Receptor Design Affects Adoptive T Cell Therapy.J Cell Physiol. 2016 Dec;231(12):2590-8. doi: 10.1002/jcp.25419. Epub 2016 Jun 2. J Cell Physiol. 2016. PMID: 27163336 Free PMC article. Review.
-
Challenges of creating effective chimeric antigen receptors for cancer therapy.Immunotherapy. 2013 Aug;5(8):783-5. doi: 10.2217/imt.13.71. Immunotherapy. 2013. PMID: 23902543 Free PMC article.
-
Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma.Cancer Immunol Res. 2019 Jan;7(1):100-112. doi: 10.1158/2326-6066.CIR-18-0307. Epub 2018 Nov 5. Cancer Immunol Res. 2019. PMID: 30396908 Free PMC article. Clinical Trial.
-
Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types.BMJ Open. 2017 Nov 12;7(11):e017075. doi: 10.1136/bmjopen-2017-017075. BMJ Open. 2017. PMID: 29133316 Free PMC article. Clinical Trial.
-
Convergent Evolution by Cancer and Viruses in Evading the NKG2D Immune Response.Cancers (Basel). 2020 Dec 18;12(12):3827. doi: 10.3390/cancers12123827. Cancers (Basel). 2020. PMID: 33352921 Free PMC article. Review.
References
-
- Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, et al. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–94. - PubMed
-
- Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–9. - PubMed
-
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

