The MLL recombinome of acute leukemias in 2013
- PMID: 23628958
- PMCID: PMC3826032
- DOI: 10.1038/leu.2013.135
The MLL recombinome of acute leukemias in 2013
Abstract
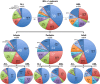
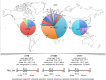
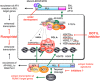
Chromosomal rearrangements of the human MLL (mixed lineage leukemia) gene are associated with high-risk infant, pediatric, adult and therapy-induced acute leukemias. We used long-distance inverse-polymerase chain reaction to characterize the chromosomal rearrangement of individual acute leukemia patients. We present data of the molecular characterization of 1590 MLL-rearranged biopsy samples obtained from acute leukemia patients. The precise localization of genomic breakpoints within the MLL gene and the involved translocation partner genes (TPGs) were determined and novel TPGs identified. All patients were classified according to their gender (852 females and 745 males), age at diagnosis (558 infant, 416 pediatric and 616 adult leukemia patients) and other clinical criteria. Combined data of our study and recently published data revealed a total of 121 different MLL rearrangements, of which 79 TPGs are now characterized at the molecular level. However, only seven rearrangements seem to be predominantly associated with illegitimate recombinations of the MLL gene (≈ 90%): AFF1/AF4, MLLT3/AF9, MLLT1/ENL, MLLT10/AF10, ELL, partial tandem duplications (MLL PTDs) and MLLT4/AF6, respectively. The MLL breakpoint distributions for all clinical relevant subtypes (gender, disease type, age at diagnosis, reciprocal, complex and therapy-induced translocations) are presented. Finally, we present the extending network of reciprocal MLL fusions deriving from complex rearrangements.
Figures


References
-
- Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. - PubMed
-
- Pui CH, Chessells JM, Camitta B, Baruchel A, Biondi A, Boyett JM, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003;17:700–706. - PubMed
-
- Szczepański T, Harrison CJ, van Dongen JJ. Genetic aberrations in paediatric acute leukaemias and implications for management of patients. Lancet Oncol. 2010;11:880–889. - PubMed
-
- Burmeister T, Marschalek R, Schneider B, Meyer C, Gökbuget N, Schwartz S, et al. Monitoring minimal residual disease by quantification of genomic chromosomal breakpoint sequences in acute leukemias with MLL aberrations. Leukemia. 2006;20:451–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

