Efavirenz-mediated induction of omeprazole metabolism is CYP2C19 genotype dependent
- PMID: 23629159
- PMCID: PMC3740059
- DOI: 10.1038/tpj.2013.17
Efavirenz-mediated induction of omeprazole metabolism is CYP2C19 genotype dependent
Abstract
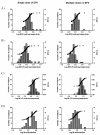
Efavirenz increases CYP2C19- and CYP3A-mediated omeprazole metabolism. We hypothesized that CYP2C19 and CYP2B6 genetic polymorphisms influence the extent of induction of omeprazole metabolism by efavirenz. Healthy subjects (n=57) were administered a single 20 mg oral dose of omeprazole on two occasions: with a single 600 mg efavirenz dose; and after a 17-day treatment with efavirenz (600 mg per day). DNA was genotyped for CYP2C19*2, *3 and *17 alleles and CYP2B6*6, *4 and *9 alleles using Taqman assays. Omeprazole, its enantiomers and metabolites were measured by liquid chromatography/tandem mass spectrometry. Our results showed that efavirenz increased omeprazole clearances in all CYP2C19 genotypes in non-stereoselective manner, but the magnitude of induction was genotype dependent. Metabolic ratios of 5-hydroxylation of omeprazole were reduced in extensive and intermediate metabolizers of CYP2C19 (P<0.05). No significant associations were observed between CYP2B6 genotypes and induction by efavirenz on omeprazole metabolism. Our data indicate how interplays between drug interactions and CYP2C19 genetic variations may influence systemic exposure of CYP2C19 substrates.
Figures

Similar articles
-
Induction of CYP2C19 and CYP3A activity following repeated administration of efavirenz in healthy volunteers.Clin Pharmacol Ther. 2012 Mar;91(3):475-82. doi: 10.1038/clpt.2011.249. Epub 2012 Feb 8. Clin Pharmacol Ther. 2012. PMID: 22318618 Free PMC article. Clinical Trial.
-
Hydroxylation index of omeprazole in relation to CYP2C19 polymorphism and sex in a healthy Iranian population.Daru. 2014 Dec 11;22(1):81. doi: 10.1186/s40199-014-0081-6. Daru. 2014. PMID: 25498969 Free PMC article. Clinical Trial.
-
Effect of co-administered inducer or inhibitor on omeprazole pharmacokinetics based on CYP2C19 genotype.J Pharmacol Sci. 2019 Apr;139(4):361-366. doi: 10.1016/j.jphs.2019.03.001. Epub 2019 Mar 11. J Pharmacol Sci. 2019. PMID: 30902567
-
Comparative pharmacokinetics of Omeprazole and its metabolites in poor and extensive metabolizer Pakistani healthy volunteers and a review of different studies.Pak J Pharm Sci. 2018 Jul;31(4):1363-1374. Pak J Pharm Sci. 2018. PMID: 30033421 Review.
-
Impact of CYP polymorphisms, ethnicity and sex differences in metabolism on dosing strategies: the case of efavirenz.Eur J Clin Pharmacol. 2014 Apr;70(4):379-89. doi: 10.1007/s00228-013-1634-1. Epub 2014 Jan 5. Eur J Clin Pharmacol. 2014. PMID: 24390631 Review.
Cited by
-
Optimising Seniors' Metabolism of Medications and Avoiding Adverse Drug Events Using Data on How Metabolism by Their P450 Enzymes Varies with Ancestry and Drug-Drug and Drug-Drug-Gene Interactions.J Pers Med. 2020 Aug 11;10(3):84. doi: 10.3390/jpm10030084. J Pers Med. 2020. PMID: 32796505 Free PMC article.
-
Pharmacological Effects and Toxicogenetic Impacts of Omeprazole: Genomic Instability and Cancer.Oxid Med Cell Longev. 2020 Mar 28;2020:3457890. doi: 10.1155/2020/3457890. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32308801 Free PMC article. Review.
-
Performance Verification of CYP2C19 Enzyme Abundance Polymorphism Settings within the Simcyp Simulator v21.Metabolites. 2022 Oct 20;12(10):1001. doi: 10.3390/metabo12101001. Metabolites. 2022. PMID: 36295903 Free PMC article.
-
Sertraline Pharmacokinetics in HIV-Infected and Uninfected Children, Adolescents, and Young Adults.Front Pediatr. 2019 Feb 6;7:16. doi: 10.3389/fped.2019.00016. eCollection 2019. Front Pediatr. 2019. PMID: 30788337 Free PMC article.
-
Evaluation of the effect of modafinil on the pharmacokinetics of encorafenib and binimetinib in patients with BRAF V600-mutant advanced solid tumors.Cancer Chemother Pharmacol. 2024 Sep;94(3):337-347. doi: 10.1007/s00280-024-04676-2. Epub 2024 Jun 15. Cancer Chemother Pharmacol. 2024. PMID: 38878209 Free PMC article. Clinical Trial.
References
-
- Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306(1):287–300. - PubMed
-
- Mutlib AE, Chen H, Nemeth GA, Markwalder JA, Seitz SP, Gan LS, et al. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab Dispos. 1999;27(11):1319–33. - PubMed
-
- Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics. 2007;8(6):547–58. - PubMed
-
- Bae SK, Jeong YJ, Lee C, Liu KH. Identification of human UGT isoforms responsible for glucuronidation of efavirenz and its three hydroxy metabolites. Xenobiotica. 41(6):437–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

