Identification of calcium binding sites on calsequestrin 1 and their implications for polymerization
- PMID: 23629537
- PMCID: PMC3719380
- DOI: 10.1039/c3mb25588c
Identification of calcium binding sites on calsequestrin 1 and their implications for polymerization
Abstract
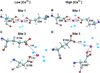
Biophysical studies have shown that each molecule of calsequestrin 1 (CASQ1) can bind about 70-80 Ca(2+) ions. However, the nature of Ca(2+)-binding sites has not yet been fully characterized. In this study, we employed in silico approaches to identify the Ca(2+) binding sites and to understand the molecular basis of CASQ1-Ca(2+) recognition. We built the protein model by extracting the atomic coordinates for the back-to-back dimeric unit from the recently solved hexameric CASQ1 structure (PDB id: ) and adding the missing C-terminal residues (aa350-364). Using this model we performed extensive 30 ns molecular dynamics simulations over a wide range of Ca(2+) concentrations ([Ca(2+)]). Our results show that the Ca(2+)-binding sites on CASQ1 differ both in affinity and geometry. The high affinity Ca(2+)-binding sites share a similar geometry and interestingly, the majority of them were found to be induced by increased [Ca(2+)]. We also found that the system shows maximal Ca(2+)-binding to the CAS (consecutive aspartate stretch at the C-terminus) before the rest of the CASQ1 surface becomes saturated. Simulated data show that the CASQ1 back-to-back stacking is progressively stabilized by the emergence of an increasing number of hydrophobic interactions with increasing [Ca(2+)]. Further, this study shows that the CAS domain assumes a compact structure with an increase in Ca(2+) binding, which suggests that the CAS domain might function as a Ca(2+)-sensor that may be a novel structural motif to sense metal. We propose the term "Dn-motif" for the CAS domain.
Figures




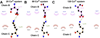


Similar articles
-
The C-terminal calcium-sensitive disordered motifs regulate isoform-specific polymerization characteristics of calsequestrin.Biopolymers. 2015 Jan;103(1):15-22. doi: 10.1002/bip.22534. Biopolymers. 2015. PMID: 25091206 Free PMC article.
-
Molecular mechanisms of pharmaceutical drug binding into calsequestrin.Int J Mol Sci. 2012 Nov 6;13(11):14326-43. doi: 10.3390/ijms131114326. Int J Mol Sci. 2012. PMID: 23203067 Free PMC article.
-
Calsequestrin 1 Is an Active Partner of Stromal Interaction Molecule 2 in Skeletal Muscle.Cells. 2021 Oct 20;10(11):2821. doi: 10.3390/cells10112821. Cells. 2021. PMID: 34831044 Free PMC article.
-
Calsequestrin: a well-known but curious protein in skeletal muscle.Exp Mol Med. 2020 Dec;52(12):1908-1925. doi: 10.1038/s12276-020-00535-1. Epub 2020 Dec 7. Exp Mol Med. 2020. PMID: 33288873 Free PMC article. Review.
-
Calsequestrin, a key protein in striated muscle health and disease.J Muscle Res Cell Motil. 2021 Jun;42(2):267-279. doi: 10.1007/s10974-020-09583-6. Epub 2020 Jun 2. J Muscle Res Cell Motil. 2021. PMID: 32488451 Review.
Cited by
-
Identification of new inhibitors against human Great wall kinase using in silico approaches.Sci Rep. 2018 Mar 20;8(1):4894. doi: 10.1038/s41598-018-23246-0. Sci Rep. 2018. PMID: 29559668 Free PMC article.
-
C-terminal residues of skeletal muscle calsequestrin are essential for calcium binding and for skeletal ryanodine receptor inhibition.Skelet Muscle. 2015 Feb 22;5:6. doi: 10.1186/s13395-015-0029-7. eCollection 2015. Skelet Muscle. 2015. PMID: 25861445 Free PMC article.
-
The impact of missense mutation in PIGA associated to paroxysmal nocturnal hemoglobinuria and multiple congenital anomalies-hypotonia-seizures syndrome 2: A computational study.Heliyon. 2019 Oct 23;5(10):e02709. doi: 10.1016/j.heliyon.2019.e02709. eCollection 2019 Oct. Heliyon. 2019. PMID: 31687525 Free PMC article.
-
Structure of RyR1 in native membranes.EMBO Rep. 2020 May 6;21(5):e49891. doi: 10.15252/embr.201949891. Epub 2020 Mar 9. EMBO Rep. 2020. PMID: 32147968 Free PMC article.
-
Calsequestrins in skeletal and cardiac muscle from adult Danio rerio.J Muscle Res Cell Motil. 2016 Apr;37(1-2):27-39. doi: 10.1007/s10974-015-9432-2. Epub 2015 Nov 20. J Muscle Res Cell Motil. 2016. PMID: 26585961
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

