The immunologically active oligosaccharides isolated from wheatgrass modulate monocytes via Toll-like receptor-2 signaling
- PMID: 23629653
- PMCID: PMC3682569
- DOI: 10.1074/jbc.M112.448381
The immunologically active oligosaccharides isolated from wheatgrass modulate monocytes via Toll-like receptor-2 signaling
Erratum in
-
The immunologically active oligosaccharides isolated from wheatgrass modulate monocytes via Toll-like receptor-2 signaling.J Biol Chem. 2015 May 8;290(19):11935. doi: 10.1074/jbc.A112.448381. J Biol Chem. 2015. PMID: 25957413 Free PMC article. No abstract available.
Abstract
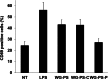
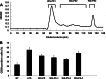
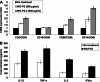
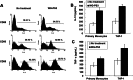
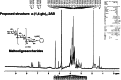
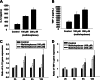
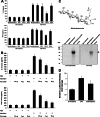
Wheatgrass is one of the most widely used health foods, but its functional components and mechanisms remain unexplored. Herein, wheatgrass-derived oligosaccharides (WG-PS3) were isolated and found to induce CD69 and Th1 cytokine expression in human peripheral blood mononuclear cells. In particular, WG-PS3 directly activated the purified monocytes by inducing the expression of CD69, CD80, CD86, IL-12, and TNF-α but affected NK and T cells only in the presence of monocytes. After further purification and structural analysis, maltoheptaose was identified from WG-PS3 as an immunomodulator. Maltoheptaose activated monocytes via Toll-like receptor 2 (TLR-2) signaling, as discovered by pretreatment of blocking antibodies against Toll-like receptors (TLRs) and also determined by click chemistry. This study is the first to reveal the immunostimulatory component of wheatgrass with well defined molecular structures and mechanisms.
Keywords: Carbohydrate Function; Immune Activation; Maltoheptaose; Monocytes; Natural Products; Oligosaccharide; Toll-like Receptors (TLR); Wheatgrass.
Figures
References
-
- Alitheen N. B., Oon C. L., Keong Y. S., Chuan T. K., Li H. K., Yong H. W. (2011) Cytotoxic effects of commercial wheatgrass and fiber towards human acute promyelocytic leukemia cells (HL60). Pak. J. Pharm. Sci. 24, 243–250 - PubMed
-
- Das A., Raychaudhuri U., Chakraborty R. (2012) Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food Sci. Nutr. 63, 718–721 - PubMed
-
- Ben-Arye E., Goldin E., Wengrower D., Stamper A., Kohn R., Berry E. (2002) Wheat grass juice in the treatment of active distal ulcerative colitis. A randomized double-blind placebo-controlled trial. Scand. J. Gastroenterol. 37, 444–449 - PubMed
-
- Shermer M. (2008) Wheatgrass juice and folk medicine. Sci. Am. 299, 42 - PubMed
-
- Chang R. (2002) Bioactive polysaccharides from traditional Chinese medicine herbs as anticancer adjuvants. J. Altern. Complement. Med. 8, 559–565 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

