Campylobacter jejuni lipooligosaccharide sialylation, phosphorylation, and amide/ester linkage modifications fine-tune human Toll-like receptor 4 activation
- PMID: 23629657
- PMCID: PMC3707672
- DOI: 10.1074/jbc.M113.468298
Campylobacter jejuni lipooligosaccharide sialylation, phosphorylation, and amide/ester linkage modifications fine-tune human Toll-like receptor 4 activation
Abstract
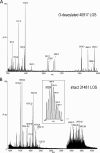
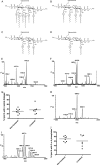
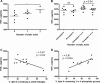
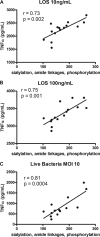
Campylobacter jejuni is a leading cause of acute gastroenteritis. C. jejuni lipooligosaccharide (LOS) is a potent activator of Toll-like receptor (TLR) 4-mediated innate immunity. Structural variations of the LOS have been previously reported in the oligosaccharide (OS) moiety, the disaccharide lipid A (LA) backbone, and the phosphorylation of the LA. Here, we studied LOS structural variation between C. jejuni strains associated with different ecological sources and analyzed their ability to activate TLR4 function. MALDI-TOF MS was performed to characterize structural variation in both the OS and LA among 15 different C. jejuni isolates. Cytokine induction in THP-1 cells and primary monocytes was correlated with LOS structural variation in each strain. Additionally, structural variation was correlated with the source of each strain. OS sialylation, increasing abundance of LA d-glucosamine versus 2,3-diamino-2,3-dideoxy-d-glucose, and phosphorylation status all correlated with TLR4 activation as measured in THP-1 cells and monocytes. Importantly, LOS-induced inflammatory responses were similar to those elicited by live bacteria, highlighting the prominent contribution of the LOS component in driving host immunity. OS sialylation status but not LA structure showed significant association with strains clustering with livestock sources. Our study highlights how variations in three structural components of C. jejuni LOS alter TLR4 activation and consequent monocyte activation.
Keywords: Campylobacter; Innate Immunity; Lipopolysaccharide (LPS); Monocytes; Toll-like Receptors (TLR).
Figures


References
-
- Allos B. M. (2001) Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32, 1201–1206 - PubMed
-
- Gardner T. J., Fitzgerald C., Xavier C., Klein R., Pruckler J., Stroika S., McLaughlin J. B. (2011) Outbreak of campylobacteriosis associated with consumption of raw peas. Clin. Infect. Dis. 53, 26–32 - PubMed
-
- Kemp R., Leatherbarrow A. J., Williams N. J., Hart C. A., Clough H. E., Turner J., Wright E. J., French N. P. (2005) Prevalence and genetic diversity of Campylobacter spp. in environmental water samples from a 100-square-kilometer predominantly dairy farming area. Appl. Environ. Microbiol. 71, 1876–1882 - PMC - PubMed
-
- Black R. E., Levine M. M., Clements M. L., Hughes T. P., Blaser M. J. (1988) Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157, 472–479 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

