Senescence marker protein-30 (SMP30) deficiency impairs myocardium-induced dilation of coronary arterioles associated with reactive oxygen species
- PMID: 23629672
- PMCID: PMC3676790
- DOI: 10.3390/ijms14059408
Senescence marker protein-30 (SMP30) deficiency impairs myocardium-induced dilation of coronary arterioles associated with reactive oxygen species
Abstract
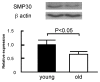
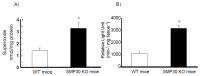
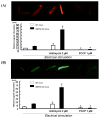
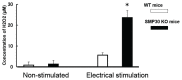
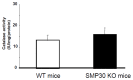
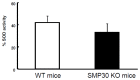
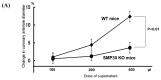
Senescence marker protein-30 (SMP30) decreases with aging. Mice with SMP30 deficiency, a model of aging, have a short lifespan with increased oxidant stress. To elucidate SMP30's effect on coronary circulation derived from myocytes, we measured the changes in the diameter of isolated coronary arterioles in wild-type (WT) mice exposed to supernatant collected from isolated paced cardiac myocytes from SMP30 KO or WT mice. Pacing increased hydrogen peroxide in myocytes, and hydrogen peroxide was greater in SMP30 KO myocytes compared to WT myocytes. Antimycin enhanced and FCCP (oxidative phosphorylation uncoupler in mitochondria) decreased superoxide production in both groups. Addition of supernatant from stimulated myocytes, either SMP30 KO or WT, caused vasodilation. The degree of the vasodilation response to supernatant was smaller in SMP30 KO mice compared to WT mice. Administration of catalase to arterioles eliminated vasodilation in myocyte supernatant of WT mice and converted vasodilation to vasoconstriction in myocyte supernatant of SMP30 KO mice. This vasoconstriction was eliminated by olmesartan, an angiotensin II receptor antagonist. Thus, SMP30 deficiency combined with oxidant stress increases angiotensin and hydrogen peroxide release from cardiac myocytes. SMP30 plays an important role in the regulation of coronary vascular tone by myocardium.
Figures



Similar articles
-
Age-related oxidant stress with senescence marker protein-30 deficiency plays a pivotal role in coronary artery spasm.Coron Artery Dis. 2013 Mar;24(2):110-8. doi: 10.1097/MCA.0b013e32835c8f96. Coron Artery Dis. 2013. PMID: 23291859
-
Aging impairs myocardium-induced dilation in coronary arterioles: role of hydrogen peroxide and angiotensin.Mech Ageing Dev. 2010 Nov-Dec;131(11-12):710-7. doi: 10.1016/j.mad.2010.09.009. Epub 2010 Oct 20. Mech Ageing Dev. 2010. PMID: 20965209
-
Metabolic regulation of coronary vascular tone: role of hydrogen peroxide, purinergic components, and angiotensin.Eur J Pharmacol. 2010 Oct 25;645(1-3):127-34. doi: 10.1016/j.ejphar.2010.07.025. Epub 2010 Jul 27. Eur J Pharmacol. 2010. PMID: 20670619
-
Involvement of senescence marker protein-30 in glucose metabolism disorder and non-alcoholic fatty liver disease.Geriatr Gerontol Int. 2016 Mar;16 Suppl 1:4-16. doi: 10.1111/ggi.12722. Geriatr Gerontol Int. 2016. PMID: 27018279 Review.
-
[Anti-aging research using SMP30/GNL knockout mice].Yakugaku Zasshi. 2010 Jan;130(1):25-8. doi: 10.1248/yakushi.130.25. Yakugaku Zasshi. 2010. PMID: 20046061 Review. Japanese.
Cited by
-
Ascorbate Is a Primary Antioxidant in Mammals.Molecules. 2022 Sep 21;27(19):6187. doi: 10.3390/molecules27196187. Molecules. 2022. PMID: 36234722 Free PMC article. Review.
-
Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries.J Mol Cell Cardiol. 2015 Jun;83:101-11. doi: 10.1016/j.yjmcc.2015.02.004. Epub 2015 Feb 7. J Mol Cell Cardiol. 2015. PMID: 25665458 Free PMC article. Review.
-
Deficiency of Senescence Marker Protein 30 Exacerbates Cardiac Injury after Ischemia/Reperfusion.Int J Mol Sci. 2016 Apr 11;17(4):542. doi: 10.3390/ijms17040542. Int J Mol Sci. 2016. PMID: 27077846 Free PMC article.
-
Effect of senescence marker protein 30 on the proliferation and apoptosis of human lens epithelial cells SRA01/04.Int J Ophthalmol. 2018 Apr 18;11(4):553-558. doi: 10.18240/ijo.2018.04.03. eCollection 2018. Int J Ophthalmol. 2018. PMID: 29675370 Free PMC article.
-
Oxidative stress in cardiovascular disease.Int J Mol Sci. 2014 Apr 9;15(4):6002-8. doi: 10.3390/ijms15046002. Int J Mol Sci. 2014. PMID: 24722571 Free PMC article.
References
-
- Sato T., Seyama K., Sato Y., Mori H., Souma S., Akiyoshi T., Kodama Y., Mori T., Goto S., Takahashi K., et al. Senescence marker protein-30 protects mice lungs from oxidative stress, aging, and smoking. Am. J. Res. Crit. Care Med. 2006;174:530–537. - PubMed
-
- Fujita T., Mandel J.L., Shirasawa T., Hino O., Shirai T., Maruyama N. Isolation of cDNA clone encoding human homologue of senescence marker protein-30 (SMP30) and its location on the X chromosome. Biochem. Biophys. Acta. 1995;1263:249–252. - PubMed
-
- Kashio A., Amano A., Kondo Y., Sakamoto T., Iwamura H., Suzuki M., Ishigami A., Yamasoba T. Effect of vitamin C depletion on age-related hearing losss in SMP30/GNL knockout mice. Biochem. Biophys. Res. Commun. 2009;390:394–398. - PubMed
-
- Son G., Kim S.J., Kim K., Kim M.S., Chung H.Y., Lee J. Cytoprotective roles of senescence marker protein 30 against intracellular calcium elevation and oxidative stress. Arch. Pharm. Res. 2008;31:872–877. - PubMed
-
- Hachamovi R., Wicker P., Cppaso J.M., Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am. J. Physiol. Heart Circ. Physiol. 1989;256:H66–H73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

